Here's pictures from last height increase update. Tibial measurement is about 16 1/4" and fibula measurement is 16". So Tibial measurement is up about 1/4" since three weeks ago. Height difference isn't significant enough yet to show up in photos so I'm going to wait a little bit longer on that. I'm taking melatonin, chondroitin + glucosamine, and starting Lithium. Melatonin is taken because it upregulates levels of TGF-Beta 1 which tells stem cells to differentiate into chondrocytes. I've noticed a lot less nerve irritation since I started chondroitin and glucosmaine which suggests that it is effective at increasing the resources available for cartilage repair(more cartilage separates the discs and makes it harder for a nerve to be impinged).
In contrast to the pictures taken a few months ago, you can see both ankles are bigger and there's a greater gap between where the calf starts to widen.
Growing Taller: How Mesenchymal Stem Cells, Microfractures, Hydrostatic Pressure, and Periosteum makes increasing height possible
Tuesday, August 31, 2010
Pax1
LSJL upregulates Pax1 2.166 fold.
Overexpression of SOX9 in mouse embryonic stem cells directs the immediate chondrogenic commitment.
Overexpression of SOX9 in mouse embryonic stem cells directs the immediate chondrogenic commitment.
"Mouse embryonic stem (mES) cells are capable of undergoing chondrogenesis in vitro. Human SOX9 (hSOX9) cDNA was delivered into mES cells. The transcripts of collagen IIA (a juvenile form), aggrecan and Pax1 were expressed in mES-hSOX9 cells grown on feeder layers, suggesting the immediate effect of exogenous SOX9 on chondrogenesis. SOX9 overexpression did not affect the cell cycle distribution in undifferentiated mES cells. Upon differentiation, collagen IIB (an adult form) was detected in day 3 immature embryoid bodies. The overexpression of exogenous SOX9 significantly induced transcriptional activity driven by SOX9 binding site. Constitutive overexpression of exogenous SOX9 in undifferentiated mES cells might have dual potentials to induce both chondrogenic commitment and growth capacity in the undifferentiated status."
"Several transcription factors are expressed in mesenchymal cells during early chondrogenesis, such as high-mobility group (HMG) box protein SOX9, and the paired-box-gene Pax-1"<-the upregulation of Pax1 by LSJL indicates that it induces early chondrogenesis.
"The expression of collagen IIB, an isoform lacking a 69 amino acid cysteine-rich domain encoded by exon 2 is specifically localized in differentiating and adult chondrocytes, indicating the differentiation into functional chondrocytes"<-LSJL did not alter COL2B expression above threshold.
Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4.
"Differentiation of mouse embryonic stem (ES) cells via embryoid bodies was established as a suitable model to study development in vitro. Differentiation of ES cells in vitro into chondrocytes can be modulated by members of the transforming growth factor-beta family (TGF-beta(1), BMP-2 and -4). Different stages of cartilage differentiation could be distinguished according to the expression pattern of the transcription factor scleraxis {LSJL upregulates scleraxis}, and the cartilage matrix protein collagen II. The number of Alcian-blue-stained areas decreased slightly after application of TGF-beta(1), whereas BMP-2 or -4 induced chondrogenic differentiation. The inducing effect of BMP-2 was found to be dependent on the time of application, consistent with its role to recruit precursor cells to the chondrogenic fate."
"Genes encoding transcription factors involved in mesenchymal differentiation, such as Pax-1 and Sox-9 as well as extracellular matrix proteins of cartilage tissue, such as collagen II and aggrecan were found to be expressed in EBs during culture"
"During early EB differentiation we found high levels of scleraxis expression in cells that did not produce collagen II and later scleraxis expressing cells were organized in nodules staining positive for Alcian blue, collagen II and COMP characteristic for developing chondrocytes"
Transcriptional control of chondrocyte fate and differentiation.
Transcriptional control of chondrocyte fate and differentiation.
"The framework of the cartilage matrix is a collagen fiber network that is comprised primarily of type II collagen (encoded by the Col2a1{up in LSJL} gene) and secondarily of type IX collagen (Col9a1{up in LSJL}, Col9a2, and Col9a3{up in LSJL}) and type XI collagen (Col11a1{up in LSJL}, Col11a2). Collagen type X (Col10a1{up in LSJL}) is produced in abundance but exclusively by prehypertrophic and hypertrophic chondrocytes."
"[Pre-cartilage condensations] express extracellular matrix and cell adhesion molecules such as N-cadherin, N-CAM (Ncam1), tenascin C (Tnc){down in LSJL}, versican{up in LSJL}, and thrombospondin-4{up in LSJL}"
"Pax1 and Pax9 are transcriptional activators with a paired box DNA-binding domain, and Nkx3.1 and Nkx3.2 are transcriptional repressors related to the Drosophila bagpipe factor."
"Expression of Pax1 and Pax9 is induced by the signaling molecule Sonic hedgehog (Shh), and expression of Nkx3.2 (possibly Nkx3.1 as well) is induced by Pax1 and Pax9"
"Nkx3.2 and Sox9 are able to induce each other's expression in the presence of Shh, and this positive autoregulatory loop can be maintained by BMP signaling and results in robust chondrogenesis"
"The homeobox transcription factor Barx2{up in LSJL} is highly expressed in precartilaginous mesenchymal condensations of the limb bud, and remains expressed in developing joints and articular cartilage"
"Atf2 transcription factor contributes to stimulate columnar chondroblast proliferation "
"Runx2 is expressed in chondrogenic mesenchymal cells [but] is no longer expressed in chondroblasts. It is reactivated, and Runx3 is activated, when chondroblasts are about to become prehypertrophic, and Runx2, but not Runx3, remains expressed through chondrocyte hypertrophy and terminal differentiation"
"Dlx5 and Dlx6 promote chondrocyte maturation and may cooperate with Runx2 and Runx3 in this function."
"Dlx5 [may] bind to a recognition site in the Col10a1 promoter and to activate a Col10a1 promoter construct"
"A negative modulator of Runx2 function is the histone deacetylase Hdac4"
The concerted action of Meox homeobox genes is required upstream of genetic pathways essential for the formation, patterning and differentiation of somites.
The concerted action of Meox homeobox genes is required upstream of genetic pathways essential for the formation, patterning and differentiation of somites.
"The induction of Pax1 and Pax9 gene expression by SHH is necessary for vertebral and rib formation"
"Meox1 mutant mice display defects restricted to sclerotomal derivatives, the vertebrae and ribs are fused"
"SHH regulates competence of cells to respond to BMP. In the absence of SHH, BMP signals result in lateral plate gene expression, but following prior exposure to SHH cells respond to BMP by inducing chondrogenesis in explant cultures"
Labels:
Pax1
Thursday, August 26, 2010
Fbxw7
F-box and WD repeat domain containing-7 (Fbxw7) Targets Endoplasmic Reticulum-Anchored Osteogenic and Chondrogenic Transcriptional Factors for Degradation.
"We recently developed a new approach, termed DiPIUS (differential proteomics-based identification of ubiquitylation substrates), for the discovery of substrates of ubiquitin ligases. We have now applied DiPIUS to Fbxw7, the F-box protein component of an SCF-type ubiquitin ligase, and thereby identified two similar transcription factors, OASIS[also known as CREB3L1 which is upregulated by LSJL] and BBF2H7[aka CREB3L2], as candidate substrates. Co-immunoprecipitation analysis confirmed that the α and γ isoforms of Fbxw7 interact with OASIS and BBF2H7 in vivo. Sustained overexpression of Fbxw7 resulted in marked down-regulation of OASIS and BBF2H7, whereas RNAi-mediated Fbxw7 depletion stabilized both proteins. Mutation of a putative Cdc4 phosphodegron in OASIS and BBF2H7 attenuated their association with Fbxw7 and resulted in their stabilization. Depletion of Fbxw7 promoted the differentiation of mouse C2C12 mesenchymal cells into osteoblasts in association with the accumulation of OASIS. Conversely, overexpression of Fbxw7 in C2C12 cells resulted in down-regulation of Col1A1 mRNA, a target of OASIS. Conditional ablation of Fbxw7 in primary mouse mesenchymal cells promoted chondrogenesis in association with up-regulation of BBF2H7, whereas overexpression of Fbxw7 inhibited chondrogenesis in ATDC5 cells. Collectively, our results suggest that OASIS and BBF2H7 are bona fide substrates of Fbxw7, and that Fbxw7 controls osteogenesis and chondrogenesis by targeting OASIS and BBF2H7, respectively, for degradation."
Sometimes Fbxw7 is pro-chondrogenic and somtimes anti-.
"BBF2H7 is highly expressed in the proliferating zone of cartilage in developing long bones"
"Type II collagen (Col2) and cartilage oligomeric matrix protein (COMP) accumulate in the ER lumen of BBF2H7-deficient chondrocytes. BBF2H7 directly activates transcription of the gene for Sec23a, a component of coat protein complex II responsible for protein transport from the ER to the Golgi apparatus, suggesting that BBF2H7 controls the secretion of extracellular matrix molecules in cartilage by regulating vesicle transport."
"The full-length (FL) forms of OASIS and BBF2H7 are cleaved in response to ER stress, and the released NH2-terminal cytoplasmic domain (N) of each protein activates the transcription of target genes. Fbxw7 exists in three isoforms (Fbxw7α, Fbxw7β, and Fbxw7γ) that differ in their NH2-terminal regions and subcellular distributions. Fbxw7α is localized to the nucleus, Fbxw7β shows a cytoplasmic distribution suggestive of localization to the ER and Golgi apparatus, and
Fbxw7γ is predominantly nucleolar, suggesting that each isoform might interact with a different set of substrates."
"Notch suppresses the differentiation and proliferation of early chondrogenic cells, suggesting that the accumulation of Notch1 induced by depletion of Fbxw7 might inhibit chondrocyte differentiation."
"We recently developed a new approach, termed DiPIUS (differential proteomics-based identification of ubiquitylation substrates), for the discovery of substrates of ubiquitin ligases. We have now applied DiPIUS to Fbxw7, the F-box protein component of an SCF-type ubiquitin ligase, and thereby identified two similar transcription factors, OASIS[also known as CREB3L1 which is upregulated by LSJL] and BBF2H7[aka CREB3L2], as candidate substrates. Co-immunoprecipitation analysis confirmed that the α and γ isoforms of Fbxw7 interact with OASIS and BBF2H7 in vivo. Sustained overexpression of Fbxw7 resulted in marked down-regulation of OASIS and BBF2H7, whereas RNAi-mediated Fbxw7 depletion stabilized both proteins. Mutation of a putative Cdc4 phosphodegron in OASIS and BBF2H7 attenuated their association with Fbxw7 and resulted in their stabilization. Depletion of Fbxw7 promoted the differentiation of mouse C2C12 mesenchymal cells into osteoblasts in association with the accumulation of OASIS. Conversely, overexpression of Fbxw7 in C2C12 cells resulted in down-regulation of Col1A1 mRNA, a target of OASIS. Conditional ablation of Fbxw7 in primary mouse mesenchymal cells promoted chondrogenesis in association with up-regulation of BBF2H7, whereas overexpression of Fbxw7 inhibited chondrogenesis in ATDC5 cells. Collectively, our results suggest that OASIS and BBF2H7 are bona fide substrates of Fbxw7, and that Fbxw7 controls osteogenesis and chondrogenesis by targeting OASIS and BBF2H7, respectively, for degradation."
Sometimes Fbxw7 is pro-chondrogenic and somtimes anti-.
"BBF2H7 is highly expressed in the proliferating zone of cartilage in developing long bones"
"Type II collagen (Col2) and cartilage oligomeric matrix protein (COMP) accumulate in the ER lumen of BBF2H7-deficient chondrocytes. BBF2H7 directly activates transcription of the gene for Sec23a, a component of coat protein complex II responsible for protein transport from the ER to the Golgi apparatus, suggesting that BBF2H7 controls the secretion of extracellular matrix molecules in cartilage by regulating vesicle transport."
"The full-length (FL) forms of OASIS and BBF2H7 are cleaved in response to ER stress, and the released NH2-terminal cytoplasmic domain (N) of each protein activates the transcription of target genes. Fbxw7 exists in three isoforms (Fbxw7α, Fbxw7β, and Fbxw7γ) that differ in their NH2-terminal regions and subcellular distributions. Fbxw7α is localized to the nucleus, Fbxw7β shows a cytoplasmic distribution suggestive of localization to the ER and Golgi apparatus, and
Fbxw7γ is predominantly nucleolar, suggesting that each isoform might interact with a different set of substrates."
"Notch suppresses the differentiation and proliferation of early chondrogenic cells, suggesting that the accumulation of Notch1 induced by depletion of Fbxw7 might inhibit chondrocyte differentiation."
Labels:
Fbxw7
Monday, August 23, 2010
CAMK4
Subcellular relocation of histone deacetylase 4 regulates growth plate chondrocyte differentiation through Ca2+/calmodulin-dependent kinase IV.
"histone deacetylase 4 (HDAC4) is located in the nucleus of chondrocytes in the proliferation zone and relocates to the cytoplasm of chondrocytes in the prehypertrophic zone in vivo. The relocation of HDAC4 from the nucleus to the cytoplasm may play a role during chondrocyte differentiation. Expression of active CaMKIV in chondrocytes promotes HDAC4 relocation into cytoplasm in primary chondrocytes. Conversely, HDAC4 relocation is blocked by a Ca(2+)/calmodulin-dependent kinase IV (CaMKIV) inhibitor. This indicates that CaMKIV signaling plays an important role in regulating HDAC4 relocation. In addition, CaMKIV is required for HDAC4 phosphorylation, which is required for HDAC4 association with the cytoplasmic protein 14-3-3. Active CaMKIV also stimulates runt-related transcription factor-2 (RunX2) and type X collagen (Col X) promoter activities and overcomes repression of these promoter activities by HDAC4. Furthermore, CaMKIV increases gene expression of the chondrocyte differentiation markers Ihh and Col X. Our results demonstrate that CaMKIV induces chondrocyte differentiation through regulation of HDAC4 subcellular relocation, from the nucleus to the cytoplasm, which results in increased activity of RunX2 and transition of chondrocytes from the proliferative to the prehypertrophic stage."
Can CAMK4 be manipulated to increase height?
"HDAC4 null mice display premature ossification of developing bones due to ectopic and early onset chondrocyte hypertrophy. This is associated with a loss of inhibition of the activity of runt-related transcription factor-2 (RunX2), a transcription factor necessary for chondrocyte hypertrophy during endochondral bone formation."
"activation of CaMKIV prevents nuclear entry of HDAC4 and enhances the binding of HDAC4 to the cytoplasmic binding protein 14-3-3 in a phosphorylation-dependent manner."
"CaMKIV is strongly expressed in prehypertrophic chondrocytes where HDAC4 is located to the cytoplasm. On the other hand, CaMKIV is weakly expressed in proliferating chondrocytes where HDAC4 is located in the nucleus. This indicates that CaMKIV may be involved in dynamic regulation of HDAC4 translocation between the cytoplasm and the nucleus in chondrocytes."
SArah M Romeim Height Increase Ally
Here's a patent that may be useful for understanding the growth plate, it's formation, and how to generation formation of new growth plates:
LIVE GROWTH PLATE CARTILAGE IMAGING TO STUDY PROLIFERATIVE COLUMN FORMATION
"The growth of long bones and the endochondral cranial base is generated by spatial and temporal control of chondrocyte maturation in the growth plate cartilage. This maturation process gives rise to domains with distinct cell morphologies and gene expression profiles. Genetic studies provide evidence that proper domain architecture is crucial to growth plate function, and this architecture is susceptible to damage via injury or disease. One important event in chondrocyte maturation is the reorganization of round precursor cells into discoid proliferative chondrocytes that form columns via clonal expansion. Previous studies support a model in which column formation by proliferative chondrocytes occurs through sequential planar division and cell intercalation in a manner similar to convergent extension{ the process by which the tissue of an embryo is restructured to converge (narrow) along one axis and extend (elongate) along a perpendicular axis by cellular movement.}. One important caveat is that these studies, and the proposed models of cell behavior in the growth plate, are based on the analysis of static images from sections of fixed tissue. The absence of dynamic information reduces confidence in the models and places limits on the mechanistic interpretation of genetic data. This proposal utilizes an in vitro live imaging system recently developed in the laboratory to elucidate the mechanisms that regulate column formation in proliferative chondrocytes. Specifically, the aims are designed to test the hypothesis that column formation occurs by cell intercalation{A type of secondary cell in which layered electrodes, usually made of metal oxides or graphite, store positive ions between the crystal layers of an electrode.} in a convergent extension process that is driven by interactions at the interface of daughter cells. This hypothesis will be tested using live imaging, together with genetic and chemical-genetic methods, to detect the presence of lamellopodia in daughter cells, to determine if actin dynamics and integrin function are required for column formation, and to determine if premature separation of daughter cells prevents cell rearrangement in Piga mutant proliferative chondrocytes. Results of these studies will provide important new information for the generation of cartilage architecture by tissue engineering and will establish this innovative method as a crucial new tool for study of the mechanisms of growth control."
No results yet but things will be very interesting once there are results.
Here's a paper on cellular poplarity she wrote. And another on Wnt signaling in cartilage.
A re-evaluation of two key reagents for in vivo studies of Wnt signaling. Although this paper doesn't have any real insight except on experimental methods.
LIVE GROWTH PLATE CARTILAGE IMAGING TO STUDY PROLIFERATIVE COLUMN FORMATION
"The growth of long bones and the endochondral cranial base is generated by spatial and temporal control of chondrocyte maturation in the growth plate cartilage. This maturation process gives rise to domains with distinct cell morphologies and gene expression profiles. Genetic studies provide evidence that proper domain architecture is crucial to growth plate function, and this architecture is susceptible to damage via injury or disease. One important event in chondrocyte maturation is the reorganization of round precursor cells into discoid proliferative chondrocytes that form columns via clonal expansion. Previous studies support a model in which column formation by proliferative chondrocytes occurs through sequential planar division and cell intercalation in a manner similar to convergent extension{ the process by which the tissue of an embryo is restructured to converge (narrow) along one axis and extend (elongate) along a perpendicular axis by cellular movement.}. One important caveat is that these studies, and the proposed models of cell behavior in the growth plate, are based on the analysis of static images from sections of fixed tissue. The absence of dynamic information reduces confidence in the models and places limits on the mechanistic interpretation of genetic data. This proposal utilizes an in vitro live imaging system recently developed in the laboratory to elucidate the mechanisms that regulate column formation in proliferative chondrocytes. Specifically, the aims are designed to test the hypothesis that column formation occurs by cell intercalation{A type of secondary cell in which layered electrodes, usually made of metal oxides or graphite, store positive ions between the crystal layers of an electrode.} in a convergent extension process that is driven by interactions at the interface of daughter cells. This hypothesis will be tested using live imaging, together with genetic and chemical-genetic methods, to detect the presence of lamellopodia in daughter cells, to determine if actin dynamics and integrin function are required for column formation, and to determine if premature separation of daughter cells prevents cell rearrangement in Piga mutant proliferative chondrocytes. Results of these studies will provide important new information for the generation of cartilage architecture by tissue engineering and will establish this innovative method as a crucial new tool for study of the mechanisms of growth control."
No results yet but things will be very interesting once there are results.
Here's a paper on cellular poplarity she wrote. And another on Wnt signaling in cartilage.
A re-evaluation of two key reagents for in vivo studies of Wnt signaling. Although this paper doesn't have any real insight except on experimental methods.
Integrin Beta4 and other integrins
Downregulation of Integrin Beta4 has been reported to inhibit chondrogenesis in TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. and upregulation was related to enhanced chondrogenesis in TiO2 nanotube stimulate chondrogenic differentiation of limb mesenchymal cells by modulating focal activity. LSJL decreases Integrin Beta 4 expression.
Integrin signaling and cell spreading alterations by rottlerin treatment of chick limb bud mesenchymal cells.
"Endochondral skeletal development begins with the formation of a cartilaginous template where mesenchymal cells aggregate and increase in density prior to their overt differentiation into chondrocytes. Prechondrogenic condensation, in which mesenchymal cells aggregate, requires cell migration and proliferation. Rottlerin suppresses migration and cell surface expression of integrin beta1 in chondrogenic progenitors. Perturbation of integrin beta1 function using an anti-integrin beta1 blocking antibody suppressed the migration of wing bud mesenchymal cells. Phosphorylation levels of Src and focal adhesion kinase (FAK) were decreased by rottlerin treatment. Cell treatment with PP2, an inhibitor of Src family kinase, or electroporation of FAK specific siRNA, suppressed cell migration in a wound-healing assay. Cells treated with rottlerin showed decreased phosphorylation of Akt, independent of PKCdelta inhibition. In addition, an Akt inhibitor suppressed the migration of chick limb bud mesenchymal cells[LSJL increases Akt-p]. Rottlerin may act as a negative regulator for cell migration, an essential step for prechondrogenic condensation, by regulating integrin beta1 signaling at focal adhesion complexes via modulation of Akt activity."
"intimate cell–cell interactions promote [mesenchymal] condensation. Condensation begins by chondroprogenitor cell–cell homophilic adhesions with an increased number of intercellular contacts occurring through gap junctions"
"Hyaluronic acid[up in LSJL], fibronectin and type I collagen[up in LSJL] all increase, and overt differentiation begins at the center of this precartilage with a subsequent increase in the production of type II collagen."
"members of the integrin family, including the α5β1 fibronectin (FN) receptor, α2β1 and α3β1, and the vitronectin receptor αvβ3 interact reversibly with both ECM proteins and cytoskeletal structures"
Integrins and extracellular matrix proteins in the human childhood and adolescent growth plate.
Integrins and extracellular matrix proteins in the human childhood and adolescent growth plate.
"[We] compare the expression of integrins (alpha1, alpha2, alpha3, alpha5, alpha6, alphav, beta1, beta3, and beta5 subunits) together with matching binding proteins in human childhood and adolescent growth plate cartilage. Integrin beta1 was detected in all chondrocytes of the growth plate cartilage, beta3 only in osteoclasts of the opening zone, and beta5 in hypertrophic chondrocytes and osteoblasts. Integrin alpha1, alpha2, and alpha5 subunits were expressed by chondrocytes in the proliferative and hypertrophic zone as well as in osteoblasts and osteoclasts. Integrin av and alpha6 subunits were present in chondrocytes of all zones, alpha3 only in osteoclasts. Collagen type II{up} and fibronectin were seen throughout the growth plate, collagen type X{up} in the hypertrophic zone, collagen type I in the ossifying trabecules. Laminin was expressed by chondrocytes in the resting zone and more weakly in the proliferative zone, collagen VI was present in the pericellular and interterritorial matrix in all zones of the growth plate. There was no difference in integrin expression in children before and during puberty. Integrin expression is not influenced by endocrine factors during sexual maturation."
Chondrosarcoma cells under mechanical strain increased expressed of a5 when adhering to fibronectin and a2 when adhering to collagen 2A1.
Chondrosarcoma cells under mechanical strain increased expressed of a5 when adhering to fibronectin and a2 when adhering to collagen 2A1.
Labels:
integrin Beta4
chondrogenesis with Smad1?
LSJL alters Smad1 expression. It likely increases it.
miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1.
"MicroRNAs (miRNA) are short non-coding RNA molecules that regulate a variety of biological processes. The role of miRNAs in BMP2-mediated biological processes is of considerable interest. A comparative miRNA array led to the isolation of several BMP2-responsive miRNAs. Among them, miR-199a(*) is of particular interest, because it was reported to be specifically expressed in the skeletal system. Here we demonstrate that miR-199a(*) is an early responsive target of BMP2: its level was dramatically reduced at 5 h, quickly increased at 24 h and remained higher thereafter in the course of BMP2-triggered chondrogenesis of a micromass culture of pluripotent C3H10T1/2 stem cells. miR-199a(*) significantly inhibited early chondrogenesis, as revealed by the reduced expression of early marker genes for chondrogenesis such as cartilage oligomeric matrix protein (COMP), type II collagen, and Sox9, whereas anti-miR-199a(*) increased the expression of these chondrogenic marker genes. A computer-based prediction algorithm led to the identification of Smad1, a well established downstream molecule of BMP-2 signaling, as a putative target of miR-199a(*). The pattern of Smad1 mRNA expression exhibited the mirror opposite of miR-199a(*) expression following BMP-2 induction. Furthermore, miR-199a(*) demonstrated remarkable inhibition of both endogenous Smad1 as well as a reporter construct bearing the 3-untranslated region of Smad1 mRNA. In addition, mutation of miR-199a(*) binding sites in the 3'-untranslated region of Smad1 mRNA abolished miR-199a(*)-mediated repression of reporter gene activity. Mechanism studies revealed that miR-199a(*) inhibits Smad1/Smad4-mediated transactivation of target genes, and that overexpression of Smad1 completely corrects miR-199a(*)-mediated repression of early chondrogenesis. Taken together, miR-199a(*) is the first BMP2 responsive microRNA found to adversely regulate early chondrocyte differentiation via direct targeting of the Smad1 transcription factor."
"Stimulation of C3H10T1/2 cells with BMP-2 resulted in the increased expression of Smad1"
Smad1 may be a pro-chondrogenic target of LSJL.
This study gives conflicting results:
The bone morphogenetic protein 2 signaling mediator Smad1 participates predominantly in osteogenic and not in chondrogenic differentiation in mesenchymal progenitors C3H10T1/2.
"The role of the bone morphogenetic protein (BMP)-signaling mediator Smad1 in osteogenic or chondrogenic differentiation was investigated in murine parental mesenchymal progenitors C3H10T1/2 and its derivatives constitutively expressing BMP-2 (C3H10T1/2-BMP-2) and, therefore, undergo BMP-mediated osteogenic/ chondrogenic development. The functions of the three Smad1 domains, that is, the N-terminal (MH1) domain, the C-terminal (MH2) domain, and the midregional proline-rich linker domain, were documented and compared with full-length Smadl. We showed that expression of the MH2 domain in parental C3H10T1/2 cells was sufficient to initiate osteogenic differentiation. Interestingly, MH1 was sufficient to initiate transcription of osteogenic marker genes like the osteocalcin or parathyroid hormone/parathyroid hormone-related protein (PTH/PTHrP) receptor. However, MH1 interfered with the histologically distinct formation of osteoblast-like cells. A dominant-negative effect on MH2-mediated osteogenic development in C3H10T1/2 cells was observed by the dose-dependent trans-expression of the midregional linker domain. Importantly, in contrast to osteogenic differentiation, Smad1 and its domains do not mimic or interfere with BMP-2-dependent chondrogenic development as monitored by the inability of MH2 to give rise to histologically distinct chondrocytes in parental C3H10T1/2 cells and by the inefficiency of the MH1 or linker domain to interfere with BMP-2-mediated chondrogenic differentiation."
miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1.
"MicroRNAs (miRNA) are short non-coding RNA molecules that regulate a variety of biological processes. The role of miRNAs in BMP2-mediated biological processes is of considerable interest. A comparative miRNA array led to the isolation of several BMP2-responsive miRNAs. Among them, miR-199a(*) is of particular interest, because it was reported to be specifically expressed in the skeletal system. Here we demonstrate that miR-199a(*) is an early responsive target of BMP2: its level was dramatically reduced at 5 h, quickly increased at 24 h and remained higher thereafter in the course of BMP2-triggered chondrogenesis of a micromass culture of pluripotent C3H10T1/2 stem cells. miR-199a(*) significantly inhibited early chondrogenesis, as revealed by the reduced expression of early marker genes for chondrogenesis such as cartilage oligomeric matrix protein (COMP), type II collagen, and Sox9, whereas anti-miR-199a(*) increased the expression of these chondrogenic marker genes. A computer-based prediction algorithm led to the identification of Smad1, a well established downstream molecule of BMP-2 signaling, as a putative target of miR-199a(*). The pattern of Smad1 mRNA expression exhibited the mirror opposite of miR-199a(*) expression following BMP-2 induction. Furthermore, miR-199a(*) demonstrated remarkable inhibition of both endogenous Smad1 as well as a reporter construct bearing the 3-untranslated region of Smad1 mRNA. In addition, mutation of miR-199a(*) binding sites in the 3'-untranslated region of Smad1 mRNA abolished miR-199a(*)-mediated repression of reporter gene activity. Mechanism studies revealed that miR-199a(*) inhibits Smad1/Smad4-mediated transactivation of target genes, and that overexpression of Smad1 completely corrects miR-199a(*)-mediated repression of early chondrogenesis. Taken together, miR-199a(*) is the first BMP2 responsive microRNA found to adversely regulate early chondrocyte differentiation via direct targeting of the Smad1 transcription factor."
"Stimulation of C3H10T1/2 cells with BMP-2 resulted in the increased expression of Smad1"
Smad1 may be a pro-chondrogenic target of LSJL.
This study gives conflicting results:
The bone morphogenetic protein 2 signaling mediator Smad1 participates predominantly in osteogenic and not in chondrogenic differentiation in mesenchymal progenitors C3H10T1/2.
"The role of the bone morphogenetic protein (BMP)-signaling mediator Smad1 in osteogenic or chondrogenic differentiation was investigated in murine parental mesenchymal progenitors C3H10T1/2 and its derivatives constitutively expressing BMP-2 (C3H10T1/2-BMP-2) and, therefore, undergo BMP-mediated osteogenic/ chondrogenic development. The functions of the three Smad1 domains, that is, the N-terminal (MH1) domain, the C-terminal (MH2) domain, and the midregional proline-rich linker domain, were documented and compared with full-length Smadl. We showed that expression of the MH2 domain in parental C3H10T1/2 cells was sufficient to initiate osteogenic differentiation. Interestingly, MH1 was sufficient to initiate transcription of osteogenic marker genes like the osteocalcin or parathyroid hormone/parathyroid hormone-related protein (PTH/PTHrP) receptor. However, MH1 interfered with the histologically distinct formation of osteoblast-like cells. A dominant-negative effect on MH2-mediated osteogenic development in C3H10T1/2 cells was observed by the dose-dependent trans-expression of the midregional linker domain. Importantly, in contrast to osteogenic differentiation, Smad1 and its domains do not mimic or interfere with BMP-2-dependent chondrogenic development as monitored by the inability of MH2 to give rise to histologically distinct chondrocytes in parental C3H10T1/2 cells and by the inefficiency of the MH1 or linker domain to interfere with BMP-2-mediated chondrogenic differentiation."
Although the other study merely suggested that Smad1 reduces miR-199 inhibition of chondrogenesis and doesn't necessarily induce it itself.
"The receptor-regulated Smads (R-Smads) Smad1, Smad5, and Smad8 are directly phosphorylated and activated by a BMP type I receptor (IA, IB, and ALK2, which is also the activin IA receptor [ActRIA])" LSJL does not affect Activin I but rather affects Activin II. LSJL also affects BMP receptor II rather than I.
"Smad2 and Smad3 are phosphorylated and activated by activin or TGF-β type I receptors (ALK4/ActRIB and ALK5/TβRI, respectively)"<-LSJL activates TGF-Beta type I receptors.
Convergence of the BMP and EGF signaling pathways on Smad1 in the regulation of chondrogenesis., also provides conflicting data. It indicates that BMP4 activates chondrogenesis by inducing the translocation of Smad4 into the nucleus and that EGF inhibits chondrogenesis by inhibiting Smad1.
Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation.
"The biological effects of type I serine/threonine kinase receptors and Smad proteins were examined using an adenovirus-based vector system. Constitutively active forms of bone morphogenetic protein (BMP) type I receptors (BMPR-IA and BMPR-IB; BMPR-I group)[LSJL induces BMP type RII] and those of activin receptor-like kinase (ALK)-1 and ALK-2 (ALK-1 group) induced alkaline phosphatase activity in C2C12 cells. Receptor-regulated Smads (R-Smads) that act in the BMP pathways, such as Smad1 and Smad5, also induced the alkaline phosphatase activity in C2C12 cells. BMP-6 dramatically enhanced alkaline phosphatase activity induced by Smad1 or Smad5, probably because of the nuclear translocation of R-Smads triggered by the ligand. Inhibitory Smads, i.e., Smad6 and Smad7, repressed the alkaline phosphatase activity induced by BMP-6 or the type I receptors. Chondrogenic differentiation of ATDC5 cells was induced by the receptors of the BMPR-I group but not by those of the ALK-1 group. However, kinase-inactive forms of the receptors of the ALK-1 and BMPR-I groups blocked chondrogenic differentiation. Although R-Smads failed to induce cartilage nodule formation, inhibitory Smads blocked it. Osteoblast differentiation induced by BMPs is thus mediated mainly via the Smad-signaling pathway, whereas chondrogenic differentiation may be transmitted by Smad-dependent and independent pathways."
"The lack of ability of ALK-1 and ALK-2 to induce differentiation of ATDC5 cells suggests that Smads may not be sufficient for chondrogenic differentiation"
Convergence of the BMP and EGF signaling pathways on Smad1 in the regulation of chondrogenesis., also provides conflicting data. It indicates that BMP4 activates chondrogenesis by inducing the translocation of Smad4 into the nucleus and that EGF inhibits chondrogenesis by inhibiting Smad1.
Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation.
"The biological effects of type I serine/threonine kinase receptors and Smad proteins were examined using an adenovirus-based vector system. Constitutively active forms of bone morphogenetic protein (BMP) type I receptors (BMPR-IA and BMPR-IB; BMPR-I group)[LSJL induces BMP type RII] and those of activin receptor-like kinase (ALK)-1 and ALK-2 (ALK-1 group) induced alkaline phosphatase activity in C2C12 cells. Receptor-regulated Smads (R-Smads) that act in the BMP pathways, such as Smad1 and Smad5, also induced the alkaline phosphatase activity in C2C12 cells. BMP-6 dramatically enhanced alkaline phosphatase activity induced by Smad1 or Smad5, probably because of the nuclear translocation of R-Smads triggered by the ligand. Inhibitory Smads, i.e., Smad6 and Smad7, repressed the alkaline phosphatase activity induced by BMP-6 or the type I receptors. Chondrogenic differentiation of ATDC5 cells was induced by the receptors of the BMPR-I group but not by those of the ALK-1 group. However, kinase-inactive forms of the receptors of the ALK-1 and BMPR-I groups blocked chondrogenic differentiation. Although R-Smads failed to induce cartilage nodule formation, inhibitory Smads blocked it. Osteoblast differentiation induced by BMPs is thus mediated mainly via the Smad-signaling pathway, whereas chondrogenic differentiation may be transmitted by Smad-dependent and independent pathways."
"The lack of ability of ALK-1 and ALK-2 to induce differentiation of ATDC5 cells suggests that Smads may not be sufficient for chondrogenic differentiation"
Labels:
smad1
Wnt pathway in chondrogenesis
WNT elements are involved very highly in LSJL. Wnt2, frillzed 2, 3, Wnt-1 inducible protein 2. Wnt's and Dkk's not enumerated.
Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation.
Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation.
"Members of the family of secreted frizzled related proteins (Sfrp) directly bind to Wnt ligands and thus preventing receptor binding"
Wnt2b has been detected (weakly) in postnatal cartilage growth plates and in mesenchymal tissues.
"Wnt5b was expressed in perichondrium, prehypertrophic and hypertrophic chondrocytes"<-Wnt5a has similar expression patterns.
"Wnt11 was expressed in the mesenchyme of developing limb buds"<-LSJL altered the frizzled receptor expression of Wnt11. Wnt11 was also expressed in prehypertrophic chondrocytes.
Sfrp 1,3, 5 were expressed in both the mesenchyme and chondrocytes(these are Wnt antagonists) It's unclear whether they were expressed during LSJL.
The nuclear hormone receptor PPARγ counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells.
The nuclear hormone receptor PPARγ counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells.
"Deletion of the nuclear receptor PPARγ in vascular smooth muscle cells of low density lipoprotein receptor (LDLr)-deficient mice fed an atherogenic diet high in cholesterol, accelerates vascular calcification with chondrogenic metaplasia within the lesions. Vascular calcification in the absence of PPARγ requires expression of the transmembrane receptor LDLr-related protein-1 in vascular smooth muscle cells. LDLr-related protein-1 promotes a previously unknown Wnt5a-dependent prochondrogenic pathway. PPARγ protects against vascular calcification by inducing the expression of secreted frizzled-related protein-2, which functions as a Wnt5a antagonist."
Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation.
"Six [Wnt's] were expressed in postnatal growth plate. Of these, Wnts -2b, -4, and -10b signal through the canonical beta-catenin pathway and Wnts -5a, -5b, and -11 signal through the noncanonical calcium pathway. The spatial expression for these six Wnts was remarkably similar, showing low mRNA expression in the resting zone, increasing expression as the chondrocytes differentiated into the proliferative and prehypertrophic state and then (except Wnt-2b) decreasing expression as the chondrocytes underwent hypertrophic differentiation. Wnt signaling modulates chondrocyte proliferation and hypertrophic differentiation. mRNA expression of these Wnt genes persisted at similar levels at 4 weeks, when longitudinal bone growth is waning."
Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation.
"Six [Wnt's] were expressed in postnatal growth plate. Of these, Wnts -2b, -4, and -10b signal through the canonical beta-catenin pathway and Wnts -5a, -5b, and -11 signal through the noncanonical calcium pathway. The spatial expression for these six Wnts was remarkably similar, showing low mRNA expression in the resting zone, increasing expression as the chondrocytes differentiated into the proliferative and prehypertrophic state and then (except Wnt-2b) decreasing expression as the chondrocytes underwent hypertrophic differentiation. Wnt signaling modulates chondrocyte proliferation and hypertrophic differentiation. mRNA expression of these Wnt genes persisted at similar levels at 4 weeks, when longitudinal bone growth is waning."
"The resting zone, adjacent to the epiphyseal bone, contains small, roughly spherical chondrocytes distributed singly or in pairs{so maybe condensation is not required to form new growth plates}, which divide infrequently and are considered "stem-like cells" capable of generating new clones of proliferative chondrocytes"
"Wnt-10a and Wnt-7b which were detected at very low levels in growth plate but not detected in bone or perichondrium"
"Genetic inactivation of β-catenin stimulates ectopic formation of chondrocytes"
"Wnt-4 overexpression accelerates hypertrophy of chondrocytes. However, mice null for Wnt-4 have been reported to have either no growth plate phenotype"
"Wnt-5a null mice display a severe skeletal phenotype with limb truncation."
"Overexpression of Wnt-11 in the developing chick limb results in slightly truncated limbs and joint fusion but does not appear to delay chondrocyte differentiation"
"Dermal fibroblasts cultured in the presence of chondroinductive demineralized bone powder show increased expression of Wnt-2b, Wnt-5b, and Wnt-10b "
"Wnts that had been readily detected in the growth plates of 1-week-old mice were still expressed in growth plate cartilage of 4-week-old mice, at similar levels."<-Wnts may not be involved in growth plate senescence.
WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways.
WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways.
"Wnt5a treatment of chondroprogenitor cells increased chondrocyte hypertrophy and was associated with an increase in nuclear factor of activated T cells (NFAT) and a decrease in nuclear factor-kappaB (NF-kappaB) activation. Wnt5a inhibited chondrocyte hypertrophy. This inhibition of hypertrophy occurred with the reciprocal signaling activation, in that a decrease in NFAT and an increase in NF-kappaB activation was observed. The increase in chondroprogenitor cell differentiation with Wnt5a treatment was blocked by calmodulin kinase or NFAT loss of function. The repression of chondrocyte hypertrophy observed was abrogated by NF-kappaB loss of function. Activation of the NFAT pathway downstream of Wnt5a negatively regulated NF-kappaB activity. Wnt5a acts to increase chondrocyte differentiation at an early stage through calmodulin kinase /NFAT-dependent induction of Sox9. Wnt5a represses chondrocyte hypertrophy via NF-kappaB-dependent inhibition of Runx2 expression."<So Wnt5a likely can help with LSJL but not with developed growth plates.
"In noncanonical Wnt calcium-dependent signaling, activation of phospholipase C through a G protein-dependent mechanism facilitates a rise in intracellular calcium concentrations. This increase in calcium then leads to the activation of several known calcium-sensitive effectors, including calmodulin kinase (CaMK), calcineurin (CaN), cAMP response element-binding protein (CREB), and protein kinase C"
"Wnt5a has been shown to specifically promote entry into the prehypertrophic phase, whereas it conversely blocks chondrocyte hypertrophy"
"nuclear factor of activated T cells (NFAT)4 [induces] chondrogenesis"<-LSJL downregulates NFAT4 as NFATC3.
The effects of Wnt inhibitors on the chondrogenesis of human mesenchymal stem cells.
The effects of Wnt inhibitors on the chondrogenesis of human mesenchymal stem cells.
"The inhibition of the Wnt pathway promotes chondrogenesis of human mesenchymal stem cells (hMSCs). In vitro pellet cultures were prepared using MSCs at passage 3 at varying concentrations of 100, 200, and 300 ng/mL of either Dickkopf (DKK)-1 or secreted frizzled-related protein (sFRP)-1, and analyzed for chondrogenic gene and protein expressions after 3 and 6 days of culture. To study the effects on chondrogenesis at a longer term, 200 ng of sFRP-1 was challenged either in the presence or absence of transforming growth factor (TGF)-beta(3) to pellets of MSCs at passage 3 for 7 days. Pellets were cultured without sFRP-1 for 14 further days. For early chondrogenesis, both DKK-1 and sFRP-1 increased glycosaminoglycan synthesis as well as the gene and protein expressions of SOX-9 and type II collagen, more prominently by sFRP-1 than by DKK-1{LSJL upregulates Dkk3}. After 21 days of in vitro chondrogenic culture under TGF-beta(3), sFRP-1 treatment did not further increase the gene expression of SOX-9 and type II collagen{this is likely the point where hypertrophic differentiation began to dominate and other transcription factors took over for Sox9}."
"Ectopic canonical Wnt signaling led to enhanced ossification and suppression of chondrocyte formation during the developmental process. Conversely, genetic inactivation of β-catenin caused ectopic formation of chondrocytes at the expense of osteoblast differentiation during both intramembranous and endochondral ossification."
"By conditionally deleting β-catenin in the limb and head mesenchyme, the osteoblast precursors lacking β-catenin were blocked from undergoing differentiation, and instead developed into chondrocytes. Their loss- and gain-of-function analyses also revealed that canonical Wnt signaling was essential for skeletal lineage differentiation, preventing transdifferentiation of osteoblastic cells into chondrocytes."
"Dickkopf (DKK)-1, inhibits Wnt signaling by binding to LRP5/6 and Kremens, a single pass transmembrane protein, forming a tertiary complex that is subsequently internalized and degraded."
"DKK-1 and sFRP-1 did not significantly affect the β-catenin gene expression. On the other hand, findings from western blotting showed that the β-catenin protein expression significantly decreased with the treatment of either DKK-1 (10%) or sFRP-1"
"When the TGF-β3 was introduced for the chondrogenic culture for longer term, sFRP-1 did not change the chondrogenic gene expression profiles. TGF-β3 overwhelmed the effect of β-catenin, making the additional treatment of Wnt inhibitors redundant."<-so it may be better to upregulate TGF-Beta than use Wnt inhibitors due to Wnt pathway's importance in hypertrophic stage of endochondral ossification.
WNT Signaling and Cartilage: Of Mice and Men.
WNT Signaling and Cartilage: Of Mice and Men.
"Chondrocytes that sparsely reside in the matrix and rarely proliferate are the key cellular mediators for cartilage homeostasis. WNT signaling pathway [is involved in] articular cartilage [maintenance]. Various WNTs are involved in the subsequent stages of chondrocyte differentiation during development, and deregulation of WNT signaling was observed in cartilage degeneration. Even though gene expression and protein synthesis can be activated upon injury, articular cartilage has a limited ability of self-repair and efforts to regenerate articular cartilage have so far not been successful. Because WNT signaling was found to be involved in the development and maintenance of cartilage as well as in the degeneration of cartilage, interfering with this pathway might contribute to improving cartilage regeneration."
Wnt pathway regulation by demineralized bone is approximated by both BMP-2 and TGF-β1 signaling.
"Allogeneic demineralized bone is used extensively as a clinical graft material because it has osteo/chondroinductive and osteoconductive properties. Demineralized bone powder (DBP) induces chondrogenic differentiation of human dermal fibroblasts (hDFs) in three-dimensional collagen cultures, but the initiating mechanisms have not been fully characterized nor has it been shown that bone morphogenetic proteins (BMPs) recapitulate DBP's effects on target cells. Among the many signaling pathways regulated in hDFs by DBP prior to in vitro chondrogenesis, there are changes in Wnts and their receptors that may contribute to DBP actions. This study tests the hypothesis that DBP modulation of Wnt signaling entails both BMP and TGF-β pathways. We compared the effects of DBP, TGF-β1, or BMP-2 on Wnt signaling components in hDFs by Wnt signaling macroarray, RT-PCR, in situ hybridization, and Western immunoblot analyses. Many effects of DBP on Wnt signaling components were not shared by BMP-2, and likewise DBP effects on Wnt genes and β-catenin only partially required the TGF-β pathway, as shown by selective inhibition of TGF-β/activin receptor-like kinase. The analyses revealed that 64% (16/25) of the Wnt signaling components regulated by DBP were regulated similarly by the sum of effects by BMP-2 and by TGF-β1. In conclusion, signaling mechanisms of inductive DBP in human dermal fibroblasts involve the modulation of multiple Wnt signals through both BMP and TGF-β pathways."
Wnt pathway regulation by demineralized bone is approximated by both BMP-2 and TGF-β1 signaling.
"Allogeneic demineralized bone is used extensively as a clinical graft material because it has osteo/chondroinductive and osteoconductive properties. Demineralized bone powder (DBP) induces chondrogenic differentiation of human dermal fibroblasts (hDFs) in three-dimensional collagen cultures, but the initiating mechanisms have not been fully characterized nor has it been shown that bone morphogenetic proteins (BMPs) recapitulate DBP's effects on target cells. Among the many signaling pathways regulated in hDFs by DBP prior to in vitro chondrogenesis, there are changes in Wnts and their receptors that may contribute to DBP actions. This study tests the hypothesis that DBP modulation of Wnt signaling entails both BMP and TGF-β pathways. We compared the effects of DBP, TGF-β1, or BMP-2 on Wnt signaling components in hDFs by Wnt signaling macroarray, RT-PCR, in situ hybridization, and Western immunoblot analyses. Many effects of DBP on Wnt signaling components were not shared by BMP-2, and likewise DBP effects on Wnt genes and β-catenin only partially required the TGF-β pathway, as shown by selective inhibition of TGF-β/activin receptor-like kinase. The analyses revealed that 64% (16/25) of the Wnt signaling components regulated by DBP were regulated similarly by the sum of effects by BMP-2 and by TGF-β1. In conclusion, signaling mechanisms of inductive DBP in human dermal fibroblasts involve the modulation of multiple Wnt signals through both BMP and TGF-β pathways."
"All three agents upregulated Wnt2{up}, Wnt5a, Fzd2{up}, Fzd7, and Gja1, and all three downregulated Msx2"
PTGS2-Upregulated in LSJL and by TGF-Beta. Downregulated by DBP and BMP-2. Col1a1 up in LSJL and upregulated by all three. Egr1 down in all three but up in LSJL.
Reduced expression of Sfrp1 during chondrogenesis and in articular chondrocytes correlates with osteoarthritis in STR/ort mice.
Reduced expression of Sfrp1 during chondrogenesis and in articular chondrocytes correlates with osteoarthritis in STR/ort mice.
"To circumvent the problems of genetic and environmental diversity hampering the analysis in humans, we turned to a murine model for human knee osteoarthritis (OA) and fine mapped a previously defined OA-quantitative trait locus (QTL). We here focused on one of the candidate genes within the OA-QTL encoding the Wnt antagonist secreted frizzled related protein 1 (Sfrp1). Sequence analysis of the Sfrp1 gene in the OA strain STR/ort revealed 23 polymorphic changes with a potential to alter the gene expression. A reduced expression in STR/ort mice was demonstrated for articular chondrocytes and hypertrophic chondrocytes of the femoral growth plate. In vitro generated mesenchymal stem cells (MSC) and chondrogenically differentiated MSC (cMSC) [had] reduced Sfrp1 expression in STR/ort mice. This reduced Sfrp1 expression in MSC correlated with an increased amount of cytoplasmic β-catenin, a downregulation of the Wnt target gene Pparγ and an upregulation of Runx2 as well as a preferential differentiation of the MSC along the osteoblasts lineage {So possibly Sfrp1 is pro-chondrogenic}. Given the role of Wnt signalling during chondrogenesis and in maintaining the integrity of the long lived articular chondrocytes, we conclude that the reduced Sfrp1 expression in STR/ort mice not only leads to an increased activation of the Wnt/ß-catenin signalling early in life but also renders the articular cartilage prone to premature ageing and to the development of OA."
"Activation and overexpression of β-catenin in adult articular chondrocytes induced hypertrophy and cell death resembling OA"
"Fgfr1{up}was shown to be expressed during MSC expansion and during chondrocyte hypertrophy in enchondral ossification and [may] be important for cell survival and extra cellular matrix production"
Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification.
Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification.
"Given their independent function, a requirement for β-catenin is not the same as that for Wnt. We investigated the effect of Wnt proteins in both tissues through generating cartilage- or bone-specific Wls null mice, respectively. Depletion of Wls by Col2-Cre, which would block Wnt secretion in the chondrocytes and perichondrium, delayed chondrocyte hypertrophy in the growth plate and impaired perichondrial osteogenesis. Loss of Wls in chondrocytes also disturbed the proliferating chondrocyte morphology and division orientation, which was similar to the defect observed in Wnt5a null mice. On the other hand, inactivation of Wls in osteoblasts by Col1-Cre resulted in a shorter hypertrophic zone and an increase of TRAP positive cell number in the chondro-osseous junction of growth plate, coupled with a decrease in bone mass. Wnt proteins not only modulate differentiation and cellular communication within populations of chondrocytes, but also mediate the cross regulation between the chondrocytes and osteoblasts in growth plate."
"CanonicalWnt/Beta-catenin signaling determines the chondrogenic or osteogenic commitment from mesenchymal progenitor cells at early stage, and promotes the hypertrophic chondrocyte differentiation at late stage"
"non-canonical Wnt5a and Wnt5b differentially regulate the transition of chondrocyte differentiation within the growth plate. The gradient of Wnt5a/PCP signaling instructs the directional limb outgrowth by establishing polarity in chondrocytes"
"Wntless (Wls), or Gpr177, is a trafficking protein that is essential for the secretion of both canonical and non-canonical Wnt proteins"
"canonical Wnt signaling is extensively active in growth plate chondrocytes"
Conditional expression of Wnt4 during chondrogenesis leads to dwarfism in mice.
Conditional expression of Wnt4 during chondrogenesis leads to dwarfism in mice.
"A mouse Wnt4 cDNA was introduced into the ubiquitously expressed Rosa26 (R26) locus by gene targeting in embryonic stem (ES) cells. The expression of Wnt4 from the R26 locus was blocked by a neomycin selection cassette flanked by loxP sites (floxneo) that was positioned between the Rosa26 promoter and the Wnt4 cDNA, creating the allele designated R26(floxneoWnt4). Wnt4 expression was activated during chondrogenesis using Col2a1-Cre transgenic mice that express Cre recombinase in differentiating chondrocytes. R26(floxneoWnt4); Col2a1-Cre double heterozygous mice exhibited a growth deficiency, beginning approximately 7 to 10 days after birth, that resulted in dwarfism. In addition, they also had craniofacial abnormalities, and delayed ossification of the lumbar vertebrae and pelvic bones. Histological analysis revealed a disruption in the organization of the growth plates and a delay in the onset of the primary and secondary ossification centers. Wnt4 overexpression caused decreased proliferation and altered maturation of chondrocytes. In addition, R26(floxneoWnt4); Col2a1-Cre mice had decreased expression of vascular endothelial growth factor (VEGF). Wnt4 overexpression leads to dwarfism in mice. Decreased VEGF expression suggests that defects in vascularization may contribute to the dwarf phenotype."
"Wnt4 accelerates chondrocyte differentiation, whereas Wnt5a inhibits this process"
"Misexpression of Wnt5b as well as Wnt5a inhibits chondrogenesis in mice, but they appear to act differently. Wnt5a inhibits the transition from resting to proliferating chondrocytes in the growth plate, whereas Wnt5b promotes this transition as well as chondrocyte proliferation"
"At 9 months of age, the tibiae of mutants were deficient in bone marrow and were filled with adipocytes in epiphyseal and metaphyseal regions. In contrast, inspection of 12-month-old control mice showed metaphyseal regions full of bone marrow"
FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells.
"members of the fibroblast growth factor (FGF), transforming growth factor-β (TGFβ) and wingless-type (Wnt) protein families are involved in controlling different differentiation stages during chondrogenesis."
"at multiple stages Smad7 inhibits chondrocyte differentiation"


"Inhibition of the p38–MAPK pathway resembles the effect of Wnt-7a misexpression in that N-cadherin expression is sustained and chondrogenesis inhibited."
"Chondrocyte maturation is reliant on an increase in Runx2 levels, accompanied by a decrease in Twist1 levels"
"disruption of Axin2 signalling-accelerated chondrocyte maturation"

"TGFβ signalling has been shown to promote cellular senescence [and stimulates proliferation as well] of MSCs through G1 arrest, achieved through activation of cyclin D kinase inhibitors (p16, p21 and p53). FGF-2, through inhibition of TGFβ2 expression, was able to suppress this cellular senescence"

"FGF-2 reduces the multipotentiality of MSCs, instead priming them for the chondrogenic lineage. "
Priming 3D cultures of human mesenchymal stromal cells towards cartilage formation via developmental pathways.
"Wnt3a and FGF2 induce hBM-MSC to proliferate in 3D culture as an undifferentiated pool of progenitors (defined by clonogenic capacity and expression of typical markers), retaining chondrogenic potential upon induction by suitable morphogens. hBM-MSC were responsive to Wnt signaling in 3D pellet culture, as assessed by significant upregulation of main target genes and increase of unphosphorylated β catenin levels. Wnt3a was able to induce a 5-fold increase in the number of proliferating hBM-MSC (6.4% vs 1.3% in the vehicle condition), although total DNA content of the 3D construct was decreasing over time. Preconditioning with Wnt3a improved TGF-β1 mediated chondrogenesis (30% more glycosaminoglycans/cell in average). In contrast to developmental and 2D MSC culture models, FGF2 antagonized the Wnt-mediated effects. Interestingly, the CD146+ subpopulation was found to be more responsive to Wnt3a."
"Upon chondrogenic and hypertrophic activation, the endochondral process proceeds in a semi-autonomous and self-regulated manner, comparable to normal embryonic development and leading to efficient hBM-MSC induction towards the osteoblastic lineage"
"Wnt exposure maintains mesenchymal cells in a proliferative state, while the exit from the signal, along the elongation of the tissue (i.e. increase in distance from Wnt source), allows for the chondrogenic differentiation to occur"
Genes activated by the Wnt pathway in the cell lines were Axin2, Dkk1, Cyclin D1, and p21. The Wnt group also had a significantly greater portion of phosphorylated Beta-Catenin. The Wnt treated group had more cells that underwent cell division.
Following TGF-Beta induction of chondrogenesis, the Wnt pretreated group was more chondrogenic than the non-treated group. Chondroinduction did not occur without TGF-Beta induction. FGF2 antagonized the beneficial affects of Wnt3a.
"the expansion of mesenchymal progenitors densely packed in 3D nodules, which precedes the complete chondrogenic differentiation of human embryonic stem cells, requires the stimulation by Wnt3a and FGF2, along with Activin-A and/or BMP4."
"hBM-MSC markedly differ from limb mesenchymal cells, so that their expansion and chondrogenesis cannot be instructed by directly recapitulating developmental cues"
FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells.
"members of the fibroblast growth factor (FGF), transforming growth factor-β (TGFβ) and wingless-type (Wnt) protein families are involved in controlling different differentiation stages during chondrogenesis."
"at multiple stages Smad7 inhibits chondrocyte differentiation"


"Inhibition of the p38–MAPK pathway resembles the effect of Wnt-7a misexpression in that N-cadherin expression is sustained and chondrogenesis inhibited."
"Chondrocyte maturation is reliant on an increase in Runx2 levels, accompanied by a decrease in Twist1 levels"
"disruption of Axin2 signalling-accelerated chondrocyte maturation"

"TGFβ signalling has been shown to promote cellular senescence [and stimulates proliferation as well] of MSCs through G1 arrest, achieved through activation of cyclin D kinase inhibitors (p16, p21 and p53). FGF-2, through inhibition of TGFβ2 expression, was able to suppress this cellular senescence"

"FGF-2 reduces the multipotentiality of MSCs, instead priming them for the chondrogenic lineage. "
Priming 3D cultures of human mesenchymal stromal cells towards cartilage formation via developmental pathways.
"Wnt3a and FGF2 induce hBM-MSC to proliferate in 3D culture as an undifferentiated pool of progenitors (defined by clonogenic capacity and expression of typical markers), retaining chondrogenic potential upon induction by suitable morphogens. hBM-MSC were responsive to Wnt signaling in 3D pellet culture, as assessed by significant upregulation of main target genes and increase of unphosphorylated β catenin levels. Wnt3a was able to induce a 5-fold increase in the number of proliferating hBM-MSC (6.4% vs 1.3% in the vehicle condition), although total DNA content of the 3D construct was decreasing over time. Preconditioning with Wnt3a improved TGF-β1 mediated chondrogenesis (30% more glycosaminoglycans/cell in average). In contrast to developmental and 2D MSC culture models, FGF2 antagonized the Wnt-mediated effects. Interestingly, the CD146+ subpopulation was found to be more responsive to Wnt3a."
"Upon chondrogenic and hypertrophic activation, the endochondral process proceeds in a semi-autonomous and self-regulated manner, comparable to normal embryonic development and leading to efficient hBM-MSC induction towards the osteoblastic lineage"
"Wnt exposure maintains mesenchymal cells in a proliferative state, while the exit from the signal, along the elongation of the tissue (i.e. increase in distance from Wnt source), allows for the chondrogenic differentiation to occur"
Genes activated by the Wnt pathway in the cell lines were Axin2, Dkk1, Cyclin D1, and p21. The Wnt group also had a significantly greater portion of phosphorylated Beta-Catenin. The Wnt treated group had more cells that underwent cell division.
Following TGF-Beta induction of chondrogenesis, the Wnt pretreated group was more chondrogenic than the non-treated group. Chondroinduction did not occur without TGF-Beta induction. FGF2 antagonized the beneficial affects of Wnt3a.
"the expansion of mesenchymal progenitors densely packed in 3D nodules, which precedes the complete chondrogenic differentiation of human embryonic stem cells, requires the stimulation by Wnt3a and FGF2, along with Activin-A and/or BMP4."
"hBM-MSC markedly differ from limb mesenchymal cells, so that their expansion and chondrogenesis cannot be instructed by directly recapitulating developmental cues"
Labels:
Wnt
Sunday, August 22, 2010
Comparison of Growth Hormone treatment with Lateral Synovial Joint Loading Histology
Under epiphyseal distraction(the method that involved gradually lengthening the epiphysis so as to not cause fracture), we see a much less dense growth plate but we see a much greater blue line(hyaline cartilage growth plate line). The distraction is not perfect as there is some ossification within the hyaline cartilage. What's important to note is that even though the white stem cells in the blue line are much less organized than under normal endochondral ossification they are still able to undergo it. Here is the relevant image: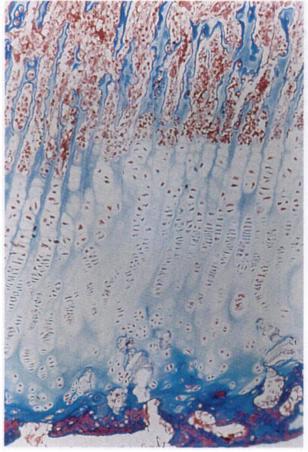
Now under LSJL(right click and click view image to see whole slide), we saw tiny dots in white(top middle) almost floating down into the growth plate(possible signs of stem cell recruitment). In contrast to epiphyseal distraction, the hypertrophic zone is denser than normal under LSJL where epiphyseal distraction decreases density in all areas. So Lateral Joint Loading has an effect beyond a stretching of the growth plate.
The study containing the slides of the growth plates under GH can be found here on page 4. The study is password protected so I was not able to copy the slides. On slide A you can see at the top of the growth plate there is a thin blue line that is the same texture as the hyaline cartilage outside the bone. Since the external blue cartilage remains as articular cartilage it is logical that the blue line of hyaline resting zone cartilage remains. Comparing slides C and D, the growth plate is much larger. In contrast to epiphyseal distraction, the resting thick blue hyaline cartilage growth plate line is not bigger. Under HGH, in contrast to LSJL, the bone quality below doesn't change. Under LSJL the bone below looking more like compact bone must be due to other factors than increasing growth rate. Otherwise the bone under LSJL is very similar to bone under HGH.

Now under LSJL(right click and click view image to see whole slide), we saw tiny dots in white(top middle) almost floating down into the growth plate(possible signs of stem cell recruitment). In contrast to epiphyseal distraction, the hypertrophic zone is denser than normal under LSJL where epiphyseal distraction decreases density in all areas. So Lateral Joint Loading has an effect beyond a stretching of the growth plate.
The study containing the slides of the growth plates under GH can be found here on page 4. The study is password protected so I was not able to copy the slides. On slide A you can see at the top of the growth plate there is a thin blue line that is the same texture as the hyaline cartilage outside the bone. Since the external blue cartilage remains as articular cartilage it is logical that the blue line of hyaline resting zone cartilage remains. Comparing slides C and D, the growth plate is much larger. In contrast to epiphyseal distraction, the resting thick blue hyaline cartilage growth plate line is not bigger. Under HGH, in contrast to LSJL, the bone quality below doesn't change. Under LSJL the bone below looking more like compact bone must be due to other factors than increasing growth rate. Otherwise the bone under LSJL is very similar to bone under HGH.
Labels:
growth hormone
Saturday, August 21, 2010
Increase Growth Hormone Levels with Niacin?
Niacin seems to be hot new thing in the height increase community despite evidence that injecting young kids with growth hormone only increases growth rate and not final adult height. Niacin taken at 100mg per day also causes skin flushing(a pretty mild side effect that causes red skin). Now, even though I don't think increasing growth hormone levels will result in an increase in final adult height, but as we saw with melatonin(another product recommended to height seekers but has effects other than increasing growth hormone like encouraging osteogenic differentiation of stem cells plus increasing chondrocyte levels of collagen II and SOX9) sometimes supplements recommended to height seekers for increasing growth hormone could have other height increasing effects.
Vitamins: not just for enzymes.
"Vitamins have traditionally played the role of coenzymes, organic molecules that facilitate the chemical reactions catalyzed by enzymes. However, several vitamins assume additional endocrine-like actions; this review will discuss four such vitamins. Vitamin K2 is involved in the gamma-carboxylation of coagulation factors and bone proteins, but it can also bind and activate the steroid and xenobiotic receptor in order to mediate transcription in bone tissue, and has been used to treat osteoporosis. Biotin is critical for some carboxylation reactions, but it also induces epidermal differentiation and has been used to treat lameness in animals and brittle nails in humans. Pyridoxal phosphate (the active form of vitamin B6) is involved in a multitude of reactions, including decarboxylation and transamination; it can also inhibit DNA polymerases and several steroid receptors and may prove useful as an adjunct in cancer chemotherapy. Finally, nicotinic acid is converted to NAD+ and NADP+, which are used as hydrogen/electron carriers in redox reactions. However, it also possesses vasodilatory and antilipolytic activities."
Vitamin K2 may be something worth looking into. Niacin could result in non-GH height increase, if NAD+ is used in any reactions that increase height(it would only make you maximum height though as you'd only use as much NAD+ as you'd need).
Extracellular NAD+: a novel autocrine/paracrine signal in osteoblast physiology.
"Intercellular communication allows co-ordination of cell metabolism and sensitivity to extracellular stimuli. In bone cells, paracrine stimulation and cell-to-cell coupling through gap junctions induce the formation of complex intercellular networks, which favours the intercellular exchange of nutrients and second messengers, ultimately controlling the process of bone remodelling. The importance of local factors in bone remodelling is known since many years. Bone cells secrete and respond to a variety signals, among which include prostaglandins, cytokines, growth factors, and ATP. We here report evidence that extracellular NAD(+) is a novel extracellular signal stimulating osteoblast differentiation. We found that HOBIT human osteoblastic cells, which are known to express ADP-ribosyl cyclase/CD38 activity, respond to micromolar concentrations of extracellular NAD(+) with oscillatory increases of the cytosolic Ca(2+) concentration. The initial Ca(2+) response was followed by a time-dependent inhibition of cell growth, the appearance of an epithelial morphology, and by an increase of alkaline phosphatase and osteocalcin expression. Under resting condition HOBIT cells release NAD(+) in the extracellular medium and the release is significantly potentiated by mechanical stimulation. Taken together these results point to NAD(+) as a novel autocrine/paracrine factor involved in stimulation and maintenance of the osteoblast differentiated phenotype."
Now Niacin is not technically the same as exogenous NAD+ as the negative feedback loop can stop Niacin from increasing NAD+ levels. Niacin also increases level of ADP-Ribose. Niacin could also help increase the GH gain from exercise.
The growth hormone response to repeated bouts of sprint exercise with and without suppression of lipolysis in men.
"A single 30-s sprint is a potent physiological stimulus for growth hormone (GH) release. However, repeated bouts of sprinting attenuate the GH response, possibly due to negative feedback via elevated systemic free fatty acids (FFA). The aim of the study was to use nicotinic acid (NA) to suppress lipolysis to investigate whether serum FFA can modulate the GH response to exercise. Seven nonobese, healthy men performed two trials, consisting of two maximal 30-s cycle ergometer sprints separated by 4 h of recovery. In one trial (NA), participants ingested NA (1 g 60 min before, and 0.5 g 60 and 180 min after sprint 1); the other was a control (Con) trial. Serum FFA was not significantly different between trials before sprint 1 but was significantly lower in the NA trial immediately before sprint 2 [NA vs. Con: mean (SD); 0.08 (0.05) vs. 0.75 (0.34) mmol/l, P < 0.05]. Peak and integrated GH were significantly greater following sprint 2 compared with sprint 1 in the NA trial [peak GH: 23.3 (7.0) vs. 7.7 (11.9) microg/l, P < 0.05; integrated GH: 1,076 (350) vs. 316 (527) microg.l(-1).60 min(-1), P < 0.05] and compared with sprint 2 in the Con trial [peak GH: 23.3 (7.0) vs. 5.2 (2.3) microg/l, P < 0.05; integrated GH: 1,076 (350) vs. 206 (118) microg.l(-1).60 min(-1), P < 0.05]. In conclusion, suppressing lipolysis resulted in a significantly greater GH response to the second of two sprints, suggesting a potential role for serum FFA in negative feedback control of the GH response to repeated exercise."
Now again Nitonic Acid or NAD+ is not the same as Niacin but it is increased by Niacin. We don't know whether Niacin will have this same effect. Here's the study cited on Niacin increasing Growth Hormone Levels:
Growth hormone, cortisol, and glucagon concentrations during plasma free fatty acid depression: different effects of nicotinic acid and an adenosine derivative (BM 11.189).
"Two chemically unrelated inhibitors of lipolysis were used in order to differentiate between the effect of FFA depression and a possible FFA-unrelated drug effect, respectively, on the plasma concentrations of GH, cortisol, and glucagon. Saline infusion served as a control experiment. In eight healthy male volunteers, a similar FFA depression by either iv infusion of nicotinic acid (3-pyridine-carboxylic acid, NA) or oral intake of an adenosine derivative, N(6)-allyl-N(6)-cyclohexyl-adenosine (AD-D), was followed by a significant GH increase (to 22.1 +/- 6.2 and 9.6 +/- 2.9 ng/ml at 240 and 270 min, respectively). Due to the large scatter of the GH concentrations during NA infusion, these responses were not significantly different. No GH increase occurred when the FFA depression was prevented by addition of a lipid infusion. In contrast, plasma cortisol and glucagon both increased significantly (by 107.4 micrograms/liter at 270 min and by 48.4 pg/ml at 60 min, respectively) during NA- but not during AD-D-induced FFA depression. Addition of the lipid infusion abolished the cortisol increase during NA infusion but had no influence on basal cortisol concentrations during AD-D intake. It lowered glucagon to values slightly below basal concentrations when added to the NA infusion and more markedly during AD-D administration. The results provide evidence that 1) depression of plasma FFA per se stimulates the secretion of GH, and 2) the increase of cortisol and glucagon during NA infusion is probably unrelated to the FFA depression. Hence, the stimulatory effect of FFA lack on glucagon secretion needs to be reconsidered."
Now remember iv infusion of nitonic acid was used not directly Niacin. But Nad+ lowers free fatty acid levels which stimulates growth hormone. Niacin has been shown to decrease free fatty acid levels so even though Niacin may not be as effective as Nad+ directly, it is still effective. Growth Hormone may increase height in bone ends covered by periosteum like the vertebrae. Here's a study that shows that growth hormone may increase growth from the periosteum:
Effects of local administration of growth hormone in peri-implant bone: an experimental study with implants in rabbit tibiae.
"PURPOSE: The objective of this study was to evaluate the qualitative and quantitative differences that could appear in newly formed peri-implant bone around Screw-Vent implants placed in rabbit tibiae when treated with local administration of growth hormone (GH).
MATERIALS AND METHODS: Eight New Zealand rabbits were randomly divided into 2 groups: the experimental group, which received 4 IU of GH in the form of lyophilized powder added to the ostectomy site before implant placement, and the control group, which did not receive GH before implant placement. Animals were sacrificed 2 weeks later, and histologic sections were obtained for histomorphometry and observation under light microscopy.
RESULTS: The sections in the GH-treated group presented enhanced growth of new trabeculae from the periosteal tissue, and the bone-to-implant contact in the experimental group was significantly greater (P < .05).
DISCUSSION: Local administration of GH stimulated a more dramatic effect than that seen previously with systemic GH administration, prompting growth from both the periosteum and endosteum.
CONCLUSIONS: Local administration of GH at the time of implant placement could enhance peri-implant bone reaction."
The Fibrous Capsule is similar to the periosteum so growth hormones ability to stimulate the periosteum may have some supplementary effect on long bone growth as well. Niacin is also known as Vitamin B3 and is easily available such as here:TwinLab Niacin (B-3) Capsules, 1000 mg, 100-Count Bottles (Pack of 3) .
.
Vitamins: not just for enzymes.
"Vitamins have traditionally played the role of coenzymes, organic molecules that facilitate the chemical reactions catalyzed by enzymes. However, several vitamins assume additional endocrine-like actions; this review will discuss four such vitamins. Vitamin K2 is involved in the gamma-carboxylation of coagulation factors and bone proteins, but it can also bind and activate the steroid and xenobiotic receptor in order to mediate transcription in bone tissue, and has been used to treat osteoporosis. Biotin is critical for some carboxylation reactions, but it also induces epidermal differentiation and has been used to treat lameness in animals and brittle nails in humans. Pyridoxal phosphate (the active form of vitamin B6) is involved in a multitude of reactions, including decarboxylation and transamination; it can also inhibit DNA polymerases and several steroid receptors and may prove useful as an adjunct in cancer chemotherapy. Finally, nicotinic acid is converted to NAD+ and NADP+, which are used as hydrogen/electron carriers in redox reactions. However, it also possesses vasodilatory and antilipolytic activities."
Vitamin K2 may be something worth looking into. Niacin could result in non-GH height increase, if NAD+ is used in any reactions that increase height(it would only make you maximum height though as you'd only use as much NAD+ as you'd need).
Extracellular NAD+: a novel autocrine/paracrine signal in osteoblast physiology.
"Intercellular communication allows co-ordination of cell metabolism and sensitivity to extracellular stimuli. In bone cells, paracrine stimulation and cell-to-cell coupling through gap junctions induce the formation of complex intercellular networks, which favours the intercellular exchange of nutrients and second messengers, ultimately controlling the process of bone remodelling. The importance of local factors in bone remodelling is known since many years. Bone cells secrete and respond to a variety signals, among which include prostaglandins, cytokines, growth factors, and ATP. We here report evidence that extracellular NAD(+) is a novel extracellular signal stimulating osteoblast differentiation. We found that HOBIT human osteoblastic cells, which are known to express ADP-ribosyl cyclase/CD38 activity, respond to micromolar concentrations of extracellular NAD(+) with oscillatory increases of the cytosolic Ca(2+) concentration. The initial Ca(2+) response was followed by a time-dependent inhibition of cell growth, the appearance of an epithelial morphology, and by an increase of alkaline phosphatase and osteocalcin expression. Under resting condition HOBIT cells release NAD(+) in the extracellular medium and the release is significantly potentiated by mechanical stimulation. Taken together these results point to NAD(+) as a novel autocrine/paracrine factor involved in stimulation and maintenance of the osteoblast differentiated phenotype."
Now Niacin is not technically the same as exogenous NAD+ as the negative feedback loop can stop Niacin from increasing NAD+ levels. Niacin also increases level of ADP-Ribose. Niacin could also help increase the GH gain from exercise.
The growth hormone response to repeated bouts of sprint exercise with and without suppression of lipolysis in men.
"A single 30-s sprint is a potent physiological stimulus for growth hormone (GH) release. However, repeated bouts of sprinting attenuate the GH response, possibly due to negative feedback via elevated systemic free fatty acids (FFA). The aim of the study was to use nicotinic acid (NA) to suppress lipolysis to investigate whether serum FFA can modulate the GH response to exercise. Seven nonobese, healthy men performed two trials, consisting of two maximal 30-s cycle ergometer sprints separated by 4 h of recovery. In one trial (NA), participants ingested NA (1 g 60 min before, and 0.5 g 60 and 180 min after sprint 1); the other was a control (Con) trial. Serum FFA was not significantly different between trials before sprint 1 but was significantly lower in the NA trial immediately before sprint 2 [NA vs. Con: mean (SD); 0.08 (0.05) vs. 0.75 (0.34) mmol/l, P < 0.05]. Peak and integrated GH were significantly greater following sprint 2 compared with sprint 1 in the NA trial [peak GH: 23.3 (7.0) vs. 7.7 (11.9) microg/l, P < 0.05; integrated GH: 1,076 (350) vs. 316 (527) microg.l(-1).60 min(-1), P < 0.05] and compared with sprint 2 in the Con trial [peak GH: 23.3 (7.0) vs. 5.2 (2.3) microg/l, P < 0.05; integrated GH: 1,076 (350) vs. 206 (118) microg.l(-1).60 min(-1), P < 0.05]. In conclusion, suppressing lipolysis resulted in a significantly greater GH response to the second of two sprints, suggesting a potential role for serum FFA in negative feedback control of the GH response to repeated exercise."
Now again Nitonic Acid or NAD+ is not the same as Niacin but it is increased by Niacin. We don't know whether Niacin will have this same effect. Here's the study cited on Niacin increasing Growth Hormone Levels:
Growth hormone, cortisol, and glucagon concentrations during plasma free fatty acid depression: different effects of nicotinic acid and an adenosine derivative (BM 11.189).
"Two chemically unrelated inhibitors of lipolysis were used in order to differentiate between the effect of FFA depression and a possible FFA-unrelated drug effect, respectively, on the plasma concentrations of GH, cortisol, and glucagon. Saline infusion served as a control experiment. In eight healthy male volunteers, a similar FFA depression by either iv infusion of nicotinic acid (3-pyridine-carboxylic acid, NA) or oral intake of an adenosine derivative, N(6)-allyl-N(6)-cyclohexyl-adenosine (AD-D), was followed by a significant GH increase (to 22.1 +/- 6.2 and 9.6 +/- 2.9 ng/ml at 240 and 270 min, respectively). Due to the large scatter of the GH concentrations during NA infusion, these responses were not significantly different. No GH increase occurred when the FFA depression was prevented by addition of a lipid infusion. In contrast, plasma cortisol and glucagon both increased significantly (by 107.4 micrograms/liter at 270 min and by 48.4 pg/ml at 60 min, respectively) during NA- but not during AD-D-induced FFA depression. Addition of the lipid infusion abolished the cortisol increase during NA infusion but had no influence on basal cortisol concentrations during AD-D intake. It lowered glucagon to values slightly below basal concentrations when added to the NA infusion and more markedly during AD-D administration. The results provide evidence that 1) depression of plasma FFA per se stimulates the secretion of GH, and 2) the increase of cortisol and glucagon during NA infusion is probably unrelated to the FFA depression. Hence, the stimulatory effect of FFA lack on glucagon secretion needs to be reconsidered."
Now remember iv infusion of nitonic acid was used not directly Niacin. But Nad+ lowers free fatty acid levels which stimulates growth hormone. Niacin has been shown to decrease free fatty acid levels so even though Niacin may not be as effective as Nad+ directly, it is still effective. Growth Hormone may increase height in bone ends covered by periosteum like the vertebrae. Here's a study that shows that growth hormone may increase growth from the periosteum:
Effects of local administration of growth hormone in peri-implant bone: an experimental study with implants in rabbit tibiae.
"PURPOSE: The objective of this study was to evaluate the qualitative and quantitative differences that could appear in newly formed peri-implant bone around Screw-Vent implants placed in rabbit tibiae when treated with local administration of growth hormone (GH).
MATERIALS AND METHODS: Eight New Zealand rabbits were randomly divided into 2 groups: the experimental group, which received 4 IU of GH in the form of lyophilized powder added to the ostectomy site before implant placement, and the control group, which did not receive GH before implant placement. Animals were sacrificed 2 weeks later, and histologic sections were obtained for histomorphometry and observation under light microscopy.
RESULTS: The sections in the GH-treated group presented enhanced growth of new trabeculae from the periosteal tissue, and the bone-to-implant contact in the experimental group was significantly greater (P < .05).
DISCUSSION: Local administration of GH stimulated a more dramatic effect than that seen previously with systemic GH administration, prompting growth from both the periosteum and endosteum.
CONCLUSIONS: Local administration of GH at the time of implant placement could enhance peri-implant bone reaction."
The Fibrous Capsule is similar to the periosteum so growth hormones ability to stimulate the periosteum may have some supplementary effect on long bone growth as well. Niacin is also known as Vitamin B3 and is easily available such as here:TwinLab Niacin (B-3) Capsules, 1000 mg, 100-Count Bottles (Pack of 3)
Labels:
Niacin
Increase disc height with GDF-5 and BMP-7
GDF-5 stands for Growth Differentiation Factor Number 5 and is encoded by the gene of the same name. It is a regulator of cell growth. BMP-7(Bone Morphogenic Protein 7) is also known as Osteogenic Protein -1 or OP-1. Like GDF-5, it is encoded by the gene of the same name BMP7. BMP-7 plays an important part of transforming MSCs into cartilage and bone. Generally, cartilage increasing height in the long bones and bone increasing height in other types of bones.
[Study progress of growth differentiation factor 5 or osteogenic protein 1[BMP-7] injection into a degenerated disc]
"To review the advance in the experimental studies and evaluate the potential therapeutic application of the growth differentiation factor 5(GDF-5) and osteogenic protein 1 (OP-1) in intervertebral disc degeneration. The growth factor was one of the most potential proteins in curing the intervertebral disc degeneration. In vitro[In an actual organism where compounds are subject to negative feedback], exogenous GDF-5 or OP-1 increased the deoxyribonucleic acid and proteoglycan contents of both nucleus pulposus and annlus fibrosis cells types significantly[nucleus pulposus is what reduces disc compression so possible increase in height]. GDF-5 at 200 ng/mL or OP-1 significantly stimulated proteoglycan synthesis and collagen synthesis[Collagen is what determines bone elasticity, which could result in more stretchable bones]. In vivo, the injection of GDF-5 (100 microg) or OP-1(100 microg in 10 microL 5% lactose) resulted in a restoration of disc height, improvement of magnetic resonance imaging scores, and histologic grading scores had statistical significance. A single injection of GDF-5 or OP-1 has a reparative capacity on intervertebral discs, presumably based on its effect to stimulate matrix metabolism of intervertebral disc cells and enhance extracellular matrix production."
So, GDF-5 and BMP-7 can reduce height loss due to intervertebral disc degeneration.
The effect of recombinant human osteogenic protein-1 on growth plate repair in a sheep model.
"Injuries to the growth plate in children can result in bone bridge formation, which ultimately lead to limb length and angular deformities. The histological and molecular changes associated with growth plate repair following the Langenskiöld procedure, a surgical technique used to remove impeding bone bridges, in conjunction with administration of recombinant human osteogenic protein-1 (rhOP-1) were examined using a sheep model. Following treatment with rhOP-1 there was an increase in the height of the growth plate immediately adjacent to the defect compared to untreated animals[But the growth plate heights of the non-defective areas of the growth plate remained the same]. The expression of type I collagen, osteopontin and decorin were observed in the growth plate adjacent to the defect in the untreated animals at day 56, but this response was accelerated in the rhOP-1 treated animals, with these molecules seen as early as day 7. Therefore, treatment with rhOP-1 initiated a complex response that was both chondrogenic and osteogenic in nature."
BMP-7 increases growth rate definitely but it could result in an increase in final height if it increased chondrocyte proliferative capacity.
"In vitro studies in rat calvarial cells, ATDC5 cells, mouse embryonic long bone cultures, transplanted perichondrial cultures and articular cartilage cultures have reported an increase in the synthesis of type I1 collagen and proteoglycans following the administration of recombinant human OP-1(rhOP-I)"<-BMP-7 increases synthesis of Type II(Cartilage) Collagen and Proteoglycans
"The increase in type I1 collagen and proteoglycan synthesis is paralleled with an increase in the mRNA expression for hyaluronan synthase-2 and CD44, molecules necessary for matrix retention"[CD44 is a receptor for hyaluronic acid] BMP-7 seems to play a role in growth plate modeling to repair defects but does not seem to induce height increase on it's own.
Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1.
"Articular cartilage is an avascular tissue with precise polarity and organization comprising 3 distinct functional zones: surface, middle, and deep. The present study was undertaken to determine the in vitro chondrogenic potential of bone morphogenetic protein 7 (BMP-7) and transforming growth factor beta1 (TGFbeta1)-induced human ESC differentiation toward the articular cartilage phenotype.
Dissociated single human ESCs were cultured and passaged on a gelatin-coated flask. The human ESCs were cultured as an aggregate in a pellet culture system for 14 days in basal chondrogenic medium (CM), CM with TGFbeta1, CM with BMP-7, or CM with both TGFbeta1 and BMP-7.
The size and wet weight of the cartilage pellets and glycosaminoglycan levels increased, with the smallest, intermediate, and greatest increases, respectively, observed with CM plus TGFbeta1 treatment, CM plus BMP-7 treatment, and CM plus TGFbeta1 and BMP-7 treatment (compared with CM treatment alone). The largest size and highest weight of the pellet was in the group in which TGFbeta1 and BMP-7 were added to the medium. However, expression of the genes for cartilage-specific aggrecan and type II collagen II, as assessed by determination of messenger RNA levels, was highest in the BMP-7-treated group. Superficial zone protein (SZP)/lubricin, a marker of the superficial zone articular chondrocyte, was not detectable under identical culture conditions.
These results demonstrate an efficient and reproducible model system of human ESC-induced chondrogenesis."
So, looking for compounds and activities that increase levels of BMP-7 is definitely worth investigating.
"The mean weight of 7 pellets per group was 1.51 mg in the CM with TGFβ1 and BMP-7 group, 1.30 mg in the CM with BMP-7 group, 0.67 mg in the CM with TGFβ1 group, and 0.40 mg in the CM alone group"<-So BMP-7 may be more chondrogenic than TGF-Beta but it could also accelerate chondrocytes towards terminal differentiation faster.
"Pellets from the CM with TGFβ1 group showed tightly packed cell density, but weak immunostaining for type II collagen. The centers of the pellets from the CM with BMP-7 group appeared undifferentiated, while their periphery showed well-differentiated chondrocytes and stained strongly for GAG and type II collagen"
New insights into BMP-7 mediated osteoblastic differentiation of primary human mesenchymal stem cells.
"BMP-7 (OP-1) is currently used clinically in revision of posterolateral spine fusions and long bone non-unions. The current study characterizes BMP-7 induced gene expression during early osteoblastic differentiation of human mesenchymal stem cells (hMSC). Primary hMSC were treated with BMP-7 for 24 or 120 h and gene expression across the entire human genome was evaluated using Affymetrix HG-U133 Plus 2.0 Arrays. 955 probe sets representing 655 genes and 95 ESTs were identified as differentially expressed and were organized into three major expression profiles (Profiles A, B and C) by hierarchical clustering. Genes from each profile were classified according to biochemical pathway analyses. Profile A, representing genes upregulated by BMP-7, revealed strong enrichment for established osteogenic marker genes, as well as several genes with undefined roles in osteoblast function, including MFI2, HAS3, ADAMTS9, HEY1, DIO2 and FGFR3. A functional screen using siRNA suggested roles for MFI2, HEY1 and DIO2 in osteoblastic differentiation of hMSC. Profile B contained genes transiently downregulated by BMP-7, including numerous genes associated with cell cycle regulation. Follow-up studies confirmed that BMP-7 attenuates cell cycle progression and cell proliferation during early osteoblastic differentiation. Profile C, comprised of genes continuously downregulated by BMP-7, exhibited strong enrichment for genes associated with chemokine/cytokine activity. Inhibitory effects of BMP-7 on cytokine secretion were verified by analysis of enriched culture media. Potent downregulation of CHI3L1, a potential biomarker for numerous joint diseases, was also observed in Profile C. A focused evaluation of BMP, GDF and BMP inhibitor expression elucidated feedback loops modulating BMP-7 bioactivity. BMP-7 was found to induce BMP-2 and downregulate GDF5 expression. Transient knockdown of BMP-2 using siRNA demonstrated that osteoinductive properties associated with BMP-7 are independent of endogenous BMP-2 expression. Noggin was identified as the predominant inhibitor induced by BMP-7 treatment.."
Genetic variation in the GDF5 region is associated with osteoarthritis, height, hip axis length and fracture risk: the Rotterdam study.
A polymorphism (rs143383; T to C) near the GDF5 gene has been associated with height.
To study the association between genetic variation in the GDF5 region and radiographic osteoarthritis (ROA) susceptibility, height, bone size parameters and fracture risk in a large population-based cohort of Caucasian elderly subjects.
6365 men and women had genotype data available. ROA was defined as a Kellgren/Lawrence (K/L) score > or =2 for hand, knee and hip joints. CTX-II levels, height, bone mineral density (BMD), bone size and fracture risk were also assessed.
rs143383 and three highly correlated single nucleotide polymorphisms (SNPs) in the GDF5 region were found to be independently associated with OA, height, bone size and fracture risk in women. Women with homozygotes for the rs143383 C allele had a 37% lower risk for hand OA (p = 8 x 10(-6)) and a 28% lower risk for knee OA (p = 0.003). In addition, they were 1.1 cm taller (p = 0.001), had a larger hip axis length (HAL) (p = 4 x 10(-4)) and had a 29% increased risk of incident non-vertebral fractures (p = 0.02). No associations with hip OA or BMD were detected. No associations were found in men.
It also replicates previous association between GDF5 variation and height. Furthermore, our findings for HAL suggest that GDF5 action is primarily directed to the long bones, rather than the axial skeleton."
"GDF5 can induce ectopic cartilage and bone formation in vivo and in vitro and is present in adult human cartilage"<-thus maybe GDF5 can induce mesenchymal chondrogenesis in the epiphyseal bone marrow.
One possibility as to why the gene has an effect on women and not on men is that it may be a sex linked trait. Of course, the fact that the trait was noticed in homozygous genes makes this possibility less likely. "sex-specific hormones, like androgens and oestrogens, might be involved in GDF5 regulation" It could be that this particular gene is not hemizygous(not functional with only one allele). Also, this gene may only interact with female specific compounds. One way to test this would be to examine the effects of GDF-5 on people who are XXY(Klinefelter's Syndrome). No such studies have yet been done.
Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells.
"We compared the standard chondrogenic protocol using transforming growth factor beta-1 (TGFß) to the effects of hypoxia, growth and differentiation factor-5 (GDF5), and coculture with bovine nucleus pulposus cells (bNPC). The efficacy of molecules recently discovered as possible nucleus pulposus (NP) markers to differentiate between chondrogenic and IVD-like differentiation was evaluated. MSCs were isolated from human bone marrow and encapsulated in alginate beads. Beads were cultured in DMEM (control) supplemented with TGFß or GDF5 or under indirect coculture with bNPC. All groups were incubated at low (2 %) or normal (20 %) oxygen tension for 28 days. Hypoxia increased aggrecan and collagen II gene expression in all groups. The hypoxic GDF5 and TGFß groups demonstrated most increased aggrecan and collagen II mRNA levels and glycosaminoglycan accumulation. Collagen I and X were most up-regulated in the TGFß groups. From the NP markers, cytokeratin-19 was expressed to highest extent in the hypoxic GDF5 groups; lowest expression was observed in the TGFß group. Levels of forkhead box F1 were down-regulated by TGFß and up-regulated by coculture with bNPC. Carbonic anhydrase 12 was also down-regulated in the TGFß group and showed highest expression in the GDF5 group cocultured with bNPC under hypoxia. Trends in gene expression regulation were confirmed on the protein level using immunohistochemistry. We conclude that hypoxia and GDF5 may be suitable for directing MSCs towards the IVD-like phenotype."
Even though GDF5 mainly directs towards intravertebral disc cells it may still help induce endochondral ossification. And interverterbral disc height influences height in the spine.
Growth differentiation factor 5 modulation of chondrogenesis of self-assembled constructs involves gap junction-mediated intercellular communication.
[Study progress of growth differentiation factor 5 or osteogenic protein 1[BMP-7] injection into a degenerated disc]
"To review the advance in the experimental studies and evaluate the potential therapeutic application of the growth differentiation factor 5(GDF-5) and osteogenic protein 1 (OP-1) in intervertebral disc degeneration. The growth factor was one of the most potential proteins in curing the intervertebral disc degeneration. In vitro[In an actual organism where compounds are subject to negative feedback], exogenous GDF-5 or OP-1 increased the deoxyribonucleic acid and proteoglycan contents of both nucleus pulposus and annlus fibrosis cells types significantly[nucleus pulposus is what reduces disc compression so possible increase in height]. GDF-5 at 200 ng/mL or OP-1 significantly stimulated proteoglycan synthesis and collagen synthesis[Collagen is what determines bone elasticity, which could result in more stretchable bones]. In vivo, the injection of GDF-5 (100 microg) or OP-1(100 microg in 10 microL 5% lactose) resulted in a restoration of disc height, improvement of magnetic resonance imaging scores, and histologic grading scores had statistical significance. A single injection of GDF-5 or OP-1 has a reparative capacity on intervertebral discs, presumably based on its effect to stimulate matrix metabolism of intervertebral disc cells and enhance extracellular matrix production."
So, GDF-5 and BMP-7 can reduce height loss due to intervertebral disc degeneration.
The effect of recombinant human osteogenic protein-1 on growth plate repair in a sheep model.
"Injuries to the growth plate in children can result in bone bridge formation, which ultimately lead to limb length and angular deformities. The histological and molecular changes associated with growth plate repair following the Langenskiöld procedure, a surgical technique used to remove impeding bone bridges, in conjunction with administration of recombinant human osteogenic protein-1 (rhOP-1) were examined using a sheep model. Following treatment with rhOP-1 there was an increase in the height of the growth plate immediately adjacent to the defect compared to untreated animals[But the growth plate heights of the non-defective areas of the growth plate remained the same]. The expression of type I collagen, osteopontin and decorin were observed in the growth plate adjacent to the defect in the untreated animals at day 56, but this response was accelerated in the rhOP-1 treated animals, with these molecules seen as early as day 7. Therefore, treatment with rhOP-1 initiated a complex response that was both chondrogenic and osteogenic in nature."
BMP-7 increases growth rate definitely but it could result in an increase in final height if it increased chondrocyte proliferative capacity.
"In vitro studies in rat calvarial cells, ATDC5 cells, mouse embryonic long bone cultures, transplanted perichondrial cultures and articular cartilage cultures have reported an increase in the synthesis of type I1 collagen and proteoglycans following the administration of recombinant human OP-1(rhOP-I)"<-BMP-7 increases synthesis of Type II(Cartilage) Collagen and Proteoglycans
"The increase in type I1 collagen and proteoglycan synthesis is paralleled with an increase in the mRNA expression for hyaluronan synthase-2 and CD44, molecules necessary for matrix retention"[CD44 is a receptor for hyaluronic acid] BMP-7 seems to play a role in growth plate modeling to repair defects but does not seem to induce height increase on it's own.
Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1.
"Articular cartilage is an avascular tissue with precise polarity and organization comprising 3 distinct functional zones: surface, middle, and deep. The present study was undertaken to determine the in vitro chondrogenic potential of bone morphogenetic protein 7 (BMP-7) and transforming growth factor beta1 (TGFbeta1)-induced human ESC differentiation toward the articular cartilage phenotype.
Dissociated single human ESCs were cultured and passaged on a gelatin-coated flask. The human ESCs were cultured as an aggregate in a pellet culture system for 14 days in basal chondrogenic medium (CM), CM with TGFbeta1, CM with BMP-7, or CM with both TGFbeta1 and BMP-7.
The size and wet weight of the cartilage pellets and glycosaminoglycan levels increased, with the smallest, intermediate, and greatest increases, respectively, observed with CM plus TGFbeta1 treatment, CM plus BMP-7 treatment, and CM plus TGFbeta1 and BMP-7 treatment (compared with CM treatment alone). The largest size and highest weight of the pellet was in the group in which TGFbeta1 and BMP-7 were added to the medium. However, expression of the genes for cartilage-specific aggrecan and type II collagen II, as assessed by determination of messenger RNA levels, was highest in the BMP-7-treated group. Superficial zone protein (SZP)/lubricin, a marker of the superficial zone articular chondrocyte, was not detectable under identical culture conditions.
These results demonstrate an efficient and reproducible model system of human ESC-induced chondrogenesis."
So, looking for compounds and activities that increase levels of BMP-7 is definitely worth investigating.
"The mean weight of 7 pellets per group was 1.51 mg in the CM with TGFβ1 and BMP-7 group, 1.30 mg in the CM with BMP-7 group, 0.67 mg in the CM with TGFβ1 group, and 0.40 mg in the CM alone group"<-So BMP-7 may be more chondrogenic than TGF-Beta but it could also accelerate chondrocytes towards terminal differentiation faster.
"Pellets from the CM with TGFβ1 group showed tightly packed cell density, but weak immunostaining for type II collagen. The centers of the pellets from the CM with BMP-7 group appeared undifferentiated, while their periphery showed well-differentiated chondrocytes and stained strongly for GAG and type II collagen"
New insights into BMP-7 mediated osteoblastic differentiation of primary human mesenchymal stem cells.
"BMP-7 (OP-1) is currently used clinically in revision of posterolateral spine fusions and long bone non-unions. The current study characterizes BMP-7 induced gene expression during early osteoblastic differentiation of human mesenchymal stem cells (hMSC). Primary hMSC were treated with BMP-7 for 24 or 120 h and gene expression across the entire human genome was evaluated using Affymetrix HG-U133 Plus 2.0 Arrays. 955 probe sets representing 655 genes and 95 ESTs were identified as differentially expressed and were organized into three major expression profiles (Profiles A, B and C) by hierarchical clustering. Genes from each profile were classified according to biochemical pathway analyses. Profile A, representing genes upregulated by BMP-7, revealed strong enrichment for established osteogenic marker genes, as well as several genes with undefined roles in osteoblast function, including MFI2, HAS3, ADAMTS9, HEY1, DIO2 and FGFR3. A functional screen using siRNA suggested roles for MFI2, HEY1 and DIO2 in osteoblastic differentiation of hMSC. Profile B contained genes transiently downregulated by BMP-7, including numerous genes associated with cell cycle regulation. Follow-up studies confirmed that BMP-7 attenuates cell cycle progression and cell proliferation during early osteoblastic differentiation. Profile C, comprised of genes continuously downregulated by BMP-7, exhibited strong enrichment for genes associated with chemokine/cytokine activity. Inhibitory effects of BMP-7 on cytokine secretion were verified by analysis of enriched culture media. Potent downregulation of CHI3L1, a potential biomarker for numerous joint diseases, was also observed in Profile C. A focused evaluation of BMP, GDF and BMP inhibitor expression elucidated feedback loops modulating BMP-7 bioactivity. BMP-7 was found to induce BMP-2 and downregulate GDF5 expression. Transient knockdown of BMP-2 using siRNA demonstrated that osteoinductive properties associated with BMP-7 are independent of endogenous BMP-2 expression. Noggin was identified as the predominant inhibitor induced by BMP-7 treatment.."
Genetic variation in the GDF5 region is associated with osteoarthritis, height, hip axis length and fracture risk: the Rotterdam study.
A polymorphism (rs143383; T to C) near the GDF5 gene has been associated with height.
To study the association between genetic variation in the GDF5 region and radiographic osteoarthritis (ROA) susceptibility, height, bone size parameters and fracture risk in a large population-based cohort of Caucasian elderly subjects.
6365 men and women had genotype data available. ROA was defined as a Kellgren/Lawrence (K/L) score > or =2 for hand, knee and hip joints. CTX-II levels, height, bone mineral density (BMD), bone size and fracture risk were also assessed.
rs143383 and three highly correlated single nucleotide polymorphisms (SNPs) in the GDF5 region were found to be independently associated with OA, height, bone size and fracture risk in women. Women with homozygotes for the rs143383 C allele had a 37% lower risk for hand OA (p = 8 x 10(-6)) and a 28% lower risk for knee OA (p = 0.003). In addition, they were 1.1 cm taller (p = 0.001), had a larger hip axis length (HAL) (p = 4 x 10(-4)) and had a 29% increased risk of incident non-vertebral fractures (p = 0.02). No associations with hip OA or BMD were detected. No associations were found in men.
It also replicates previous association between GDF5 variation and height. Furthermore, our findings for HAL suggest that GDF5 action is primarily directed to the long bones, rather than the axial skeleton."
"GDF5 can induce ectopic cartilage and bone formation in vivo and in vitro and is present in adult human cartilage"<-thus maybe GDF5 can induce mesenchymal chondrogenesis in the epiphyseal bone marrow.
One possibility as to why the gene has an effect on women and not on men is that it may be a sex linked trait. Of course, the fact that the trait was noticed in homozygous genes makes this possibility less likely. "sex-specific hormones, like androgens and oestrogens, might be involved in GDF5 regulation" It could be that this particular gene is not hemizygous(not functional with only one allele). Also, this gene may only interact with female specific compounds. One way to test this would be to examine the effects of GDF-5 on people who are XXY(Klinefelter's Syndrome). No such studies have yet been done.
Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells.
"We compared the standard chondrogenic protocol using transforming growth factor beta-1 (TGFß) to the effects of hypoxia, growth and differentiation factor-5 (GDF5), and coculture with bovine nucleus pulposus cells (bNPC). The efficacy of molecules recently discovered as possible nucleus pulposus (NP) markers to differentiate between chondrogenic and IVD-like differentiation was evaluated. MSCs were isolated from human bone marrow and encapsulated in alginate beads. Beads were cultured in DMEM (control) supplemented with TGFß or GDF5 or under indirect coculture with bNPC. All groups were incubated at low (2 %) or normal (20 %) oxygen tension for 28 days. Hypoxia increased aggrecan and collagen II gene expression in all groups. The hypoxic GDF5 and TGFß groups demonstrated most increased aggrecan and collagen II mRNA levels and glycosaminoglycan accumulation. Collagen I and X were most up-regulated in the TGFß groups. From the NP markers, cytokeratin-19 was expressed to highest extent in the hypoxic GDF5 groups; lowest expression was observed in the TGFß group. Levels of forkhead box F1 were down-regulated by TGFß and up-regulated by coculture with bNPC. Carbonic anhydrase 12 was also down-regulated in the TGFß group and showed highest expression in the GDF5 group cocultured with bNPC under hypoxia. Trends in gene expression regulation were confirmed on the protein level using immunohistochemistry. We conclude that hypoxia and GDF5 may be suitable for directing MSCs towards the IVD-like phenotype."
Even though GDF5 mainly directs towards intravertebral disc cells it may still help induce endochondral ossification. And interverterbral disc height influences height in the spine.
Growth differentiation factor 5 modulation of chondrogenesis of self-assembled constructs involves gap junction-mediated intercellular communication.
" novel scaffold-free self-assembled cartilage construct has been generated and used to repair particular chondral defects effectively. We hypothesize that gap junction intercellular communication (GJIC) plays a critical role in the development of self-assembled constructs upon GDF-5 induction. In this study, we investigated the effect of connexin 43 (C×43) mediated GJIC on GDF-5 modulation of chondrogenesis from two aspects, cell monolayer culture and 3-D self-assembly culture. We induced cells or self-assembled constructs with chondrogenic media (CM), growth differentiation factor 5 (GDF-5) or 1-heptanol for 3 weeks. At the end of that time, the results of quantitative fluorescence redistribution after photobleaching (FRAP) assay and immunofluorescence demonstrated that GDF-5 improved both GJIC and chondrogenic differentiation to a significant degree while 1-heptanol nearly offset the expected improvements in chondrogenesis. Biochemical assay and histology showed that GDF-5 can obviously enhance GAG, C×43 and type II collagen expressions. Conversely, we also showed that while 1-heptanol weakened GAG and type II collagen expression in self-assembled constructs, it had no effect on C×43 expression. Furthermore, real-time polymerase chain reaction showed that GDF-5 enhanced GAG and type II collagen transcription while 1-heptanol reduced them, but was affectless on C×43 transcription. This suggests that the generation of scaffold-free self-assembled cartilage from human mesenchymal stem cells upon GDF-5 induction may be mediated, at least in part, via the modulation of GJIC."
"[The] condensation stage of chondrogenesis is mediated by intercellular communication, most probably via gap junctions"
"contact between mesenchymal cells is necessary for the intercellular signal exchanges, which contribute to the initiation of condensation and the subsequent formation of chondroprogenitor cells."
" 100 ng/mL of GDF-5 was highly effective at inducing the differentiation of fibroblasts, periosteum-derived cells, and stem cells into chondrocytes while at the same time promoting the secretion of a chondrocyte-specific collagen and proteoglycan matrix"
Labels:
BMP-7,
GDF-5,
intervertebral discs
Friday, August 20, 2010
Does masturbation stunt height growth?
One of the common beliefs proliferated amongst individuals is that masturbation stunts height growth. Masturbation definitely has a powerful mood, altering affect. One come reason that masturbation would inhibit endochondral ossification is that it lowers your Testosterone levels. Now Testosterone is a myostatin inhibitor and myostatin inhibits stem cell proliferation. Another theory about why masturbation could inhibit growth is that masturbation uses up IGF-1 that could be used elsewhere in the body.
We know about the bodies negative feedback mechanisms. In the bodies internal law of supply and demand, the laws of demand are more important. If the body requires more testosterone, it will produce more testosterone in response. A longitudinal study would have to be done to see if masturbation resulted in a change in testosterone levels.
Some have wondered, if Chondroitin and Glucosamine can result in height increase then why haven't there been more reports of the usage of Chondroitin and Glucosamine resulting in height increase? It goes back to the supply and demand of the body, if the body isn't demanding the chondroitin and glucosamine then taking it won't do any good. Chondroitin and Glucosamine is for people who have disc degeneration and the body can't repair the discs fast enough. Chondroitin and Glucosamine gets your body the resources it needs to replenish the discs proteoglycan content.
Supplementing is most useful for things that the body doesn't produce or that the body doesn't produce enough of(but has more demand for). For getting around the bodies demand, you have to take things that inhibit the bodies negative feedback or you have to cycle the supplements to try and trick the body.
Masturbation increases your bodies demand for IGF-1 and Testosterone. To modify how your body works it's much more powerful to try to increase the bodies demand than it is to try to conserve or increase supply. Breaking down muscle is much more anabolic than eating more food.
Masturbation can alter your mental state. You can with hold from it to gain more motivation or you can perform it to relax into a deep slumber.
But otherwise Masturbation increases your bodies demand for anabolic hormones which is a good thing.
We know about the bodies negative feedback mechanisms. In the bodies internal law of supply and demand, the laws of demand are more important. If the body requires more testosterone, it will produce more testosterone in response. A longitudinal study would have to be done to see if masturbation resulted in a change in testosterone levels.
Some have wondered, if Chondroitin and Glucosamine can result in height increase then why haven't there been more reports of the usage of Chondroitin and Glucosamine resulting in height increase? It goes back to the supply and demand of the body, if the body isn't demanding the chondroitin and glucosamine then taking it won't do any good. Chondroitin and Glucosamine is for people who have disc degeneration and the body can't repair the discs fast enough. Chondroitin and Glucosamine gets your body the resources it needs to replenish the discs proteoglycan content.
Supplementing is most useful for things that the body doesn't produce or that the body doesn't produce enough of(but has more demand for). For getting around the bodies demand, you have to take things that inhibit the bodies negative feedback or you have to cycle the supplements to try and trick the body.
Masturbation increases your bodies demand for IGF-1 and Testosterone. To modify how your body works it's much more powerful to try to increase the bodies demand than it is to try to conserve or increase supply. Breaking down muscle is much more anabolic than eating more food.
Masturbation can alter your mental state. You can with hold from it to gain more motivation or you can perform it to relax into a deep slumber.
But otherwise Masturbation increases your bodies demand for anabolic hormones which is a good thing.
Labels:
masturbation and height
Thursday, August 19, 2010
Comparison of Epiphyseal Distraction versus Lateral Joint Loading
Previously, I've written about epiphyseal distraction before and how it was found to only increase growth rate and not actually increase "final" adult height. If the slides of epiphyseal distraction versus slides of rats under LSJL were compared, we could see if there were any differences like signs of mesenchymal stem cell recruitment. And if you look at Slide B, you can see tiny white cells coming down from the bone into the cartilage.
I asked Hiroki Yokota about it and he said the results are consistent throughout all mice. "This is a mouse study and the images are representative. We conducted histomorphometry (measurements of thickness, #cells, etc.) and statistical tests. Therefore, the results are evidence based and statistically significant." One thing I've noticed with inquiring experimenters is that they are willing to answer questions about experiment methodology but aren't willing to offer their opinions as to conclusions. I can understand why for fear of being quoted for their opinion to use the authority of their research status.
All the slides are from the study: "Limb Lengthening by Distraction of the Epiphyseal Plate: A comparison of two techniques in the rabbit." The study explains epiphyseal distraction: "[The method] of distracting the epiphyseal plate employs smaller forces and/or a slow rate of distraction with the intention of inducing an increase in the activity of the growth plate without causing either fracture or gap. Thus the functional integrity of the plate is maintained until the end of the physiological growth period. In 1979 De Bastiani, Aldegheri and Renzi Brivio introduced the term chondrodiatasis to describe this slow, controlled and symmetrical distraction of the epiphyseal plate without fracture or rupture."
"Group 2: chondrodiatasis. After 7 and 14 days of distraction, an increase in the height of the growth plate was observed, but the line of the growth cartilage was regular (Fig. 6). The bridging cartilage appeared to be slightly hyperplastic with modest changes in the columnar architecture limited to a few points in the epiphyseal plate. There was no evidence of detachment of the epiphyseal nucleus, nor of any haemorrhage (Fig. 7).
By day 28, at the end of the period of distraction, the lengthened portion was occupied by ossification tissue
similar in appearance to that of the metaphyseal bone of the control femur. The appearance was not uniform,
because of brownish zones which were areas of tissue undergoing ossification. The line of the cartilage showed normal morphology, but at some points it was markedly thicker. At the periphery of the plate there was an increase in the thickness of the periosteum without gaps in the perichondrium.
The bridging cartilage in the zones of increased thickness displayed marked hyperplasia and hypertrophy, with some disorganisation of the columnar structure but no evidence of cellular damage (Figs 8 to I I). Histological specimens taken on day 50 and day 70 showed an active epiphyseal plate which had returned to normal thickness (Fig. 12). Cellular morphology confirmed the normal organisation of the growth plate and adjacent elements, matching that of the control limb[The control limb was one undergoing distraction osteogenesis in the epiphysis]."

The growth plate 7 days after performing epiphyseal distraction. Can't see the incoming cells like in LSJL.

28 days after epiphyseal distraction. The blue line is the hyaline cartilage growth plate line. The red looks like blood flow but the study claimed no sign of hemorrhage. Blood flow is very anabolic though!
So, evidence of a hyaline cartilage growth plate line after "fusion" and differences between LSJL and epiphyseal distraction.
Here's a normal growth plate:

Here's the "fused" bone after distraction osteogenesis:

The blue line is still there. The idea behind LSJL is to get stem cells or periosteal progenitor cells to this blue region and then in there the stem cells will undergo differentiation into chondrocytes and endochondral ossification will begin. Here's what happened when the performed distraction osteogenesis on the growth plate cartilage:

The parts of the cartilage that turned into bone remarkably correlates with the blood splatters.
I asked Hiroki Yokota about it and he said the results are consistent throughout all mice. "This is a mouse study and the images are representative. We conducted histomorphometry (measurements of thickness, #cells, etc.) and statistical tests. Therefore, the results are evidence based and statistically significant." One thing I've noticed with inquiring experimenters is that they are willing to answer questions about experiment methodology but aren't willing to offer their opinions as to conclusions. I can understand why for fear of being quoted for their opinion to use the authority of their research status.
All the slides are from the study: "Limb Lengthening by Distraction of the Epiphyseal Plate: A comparison of two techniques in the rabbit." The study explains epiphyseal distraction: "[The method] of distracting the epiphyseal plate employs smaller forces and/or a slow rate of distraction with the intention of inducing an increase in the activity of the growth plate without causing either fracture or gap. Thus the functional integrity of the plate is maintained until the end of the physiological growth period. In 1979 De Bastiani, Aldegheri and Renzi Brivio introduced the term chondrodiatasis to describe this slow, controlled and symmetrical distraction of the epiphyseal plate without fracture or rupture."
"Group 2: chondrodiatasis. After 7 and 14 days of distraction, an increase in the height of the growth plate was observed, but the line of the growth cartilage was regular (Fig. 6). The bridging cartilage appeared to be slightly hyperplastic with modest changes in the columnar architecture limited to a few points in the epiphyseal plate. There was no evidence of detachment of the epiphyseal nucleus, nor of any haemorrhage (Fig. 7).
By day 28, at the end of the period of distraction, the lengthened portion was occupied by ossification tissue
similar in appearance to that of the metaphyseal bone of the control femur. The appearance was not uniform,
because of brownish zones which were areas of tissue undergoing ossification. The line of the cartilage showed normal morphology, but at some points it was markedly thicker. At the periphery of the plate there was an increase in the thickness of the periosteum without gaps in the perichondrium.
The bridging cartilage in the zones of increased thickness displayed marked hyperplasia and hypertrophy, with some disorganisation of the columnar structure but no evidence of cellular damage (Figs 8 to I I). Histological specimens taken on day 50 and day 70 showed an active epiphyseal plate which had returned to normal thickness (Fig. 12). Cellular morphology confirmed the normal organisation of the growth plate and adjacent elements, matching that of the control limb[The control limb was one undergoing distraction osteogenesis in the epiphysis]."

The growth plate 7 days after performing epiphyseal distraction. Can't see the incoming cells like in LSJL.

28 days after epiphyseal distraction. The blue line is the hyaline cartilage growth plate line. The red looks like blood flow but the study claimed no sign of hemorrhage. Blood flow is very anabolic though!
So, evidence of a hyaline cartilage growth plate line after "fusion" and differences between LSJL and epiphyseal distraction.
Here's a normal growth plate:

Here's the "fused" bone after distraction osteogenesis:

The blue line is still there. The idea behind LSJL is to get stem cells or periosteal progenitor cells to this blue region and then in there the stem cells will undergo differentiation into chondrocytes and endochondral ossification will begin. Here's what happened when the performed distraction osteogenesis on the growth plate cartilage:

The parts of the cartilage that turned into bone remarkably correlates with the blood splatters.
Labels:
epiphyseal distraction
Subscribe to:
Posts (Atom)

